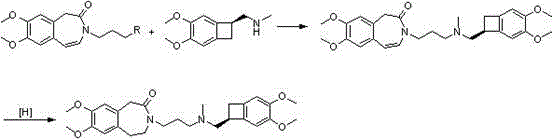
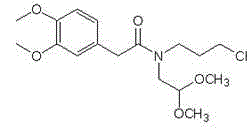
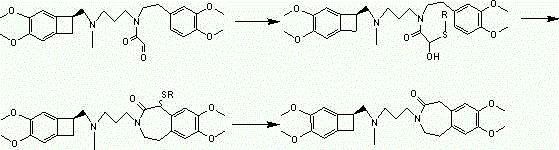
Set of intermediate compounds used for synthesis of Ivabradine, and applications thereof
A technology for ivabradine and a compound, which is applied to the application field of ivabradine synthesis, can solve the problems of high preparation cost of ivabradine, difficult to obtain starting materials, large production pollution, etc., and achieves reasonable reaction sequence, The effect of simple reaction and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation of compound II
[0049] Add 10.9mL (0.1mol) of aminoacetaldehyde dimethyl acetal, 16.6mL (0.12mol) of triethylamine, and 100mL of dichloromethane into the reaction flask and place it in an ice-water bath. Add 15.8g of 1,3-bromochloropropane ( 0.1mol) was dissolved in 50mL of dichloromethane to form a dropwise solution, and the dropwise solution was dropped into the aforementioned reaction solution placed in an ice bath, stirred and reacted for 1h, washed with 100ml of saturated sodium bicarbonate solution, washed with 100mL of water, and separated layer, the dichloromethane layer was collected, dried, and concentrated under reduced pressure to obtain 17.2 g of a colorless to light yellow oily substance (Compound II), with a yield of 95%.
Embodiment 2
[0050] Embodiment 2: the preparation of compound III
[0051] Add 17.2g (0.095mol) of compound II, 14.5ml (0.105mol) of triethylamine, and 100ml of dichloromethane into the reaction flask and place it in an ice-water bath. Add 21.4g of 3,4-dimethoxy-phenylacetyl chloride (0.1mol) was dissolved in 100mL of dichloromethane to form a dropwise solution, and the dropwise solution was dropped into the aforementioned reaction solution placed in an ice bath, stirred and reacted for 1h, washed with 100ml of saturated sodium bicarbonate solution, and washed with 100mL of water, The layers were separated, and the dichloromethane layer was collected, dried, and concentrated under reduced pressure to obtain 32.7 g of a brown-yellow oily substance (Compound III), with a yield of 96%.
Embodiment 3
[0052] Embodiment 3: the preparation of compound IV
[0053] Take compound III 10.8g (0.03mol), (1S)-4,5-dimethoxy-1-[(methylamino)methyl]benzocyclobutane hydrochloride 6.1g (0.025mol), carbonic acid Potassium 8.7g (0.063mol) and 1.50g sodium iodide 3.75g (0.025mol) were refluxed in 50mL methyl isobutyl ketone for 6h, cooled, added 50mL of 1N hydrochloric acid, separated into layers, collected the water layer, and used Sodium hydroxide was adjusted to pH = 10, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated to dryness to obtain 12.1 g of compound IV with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com