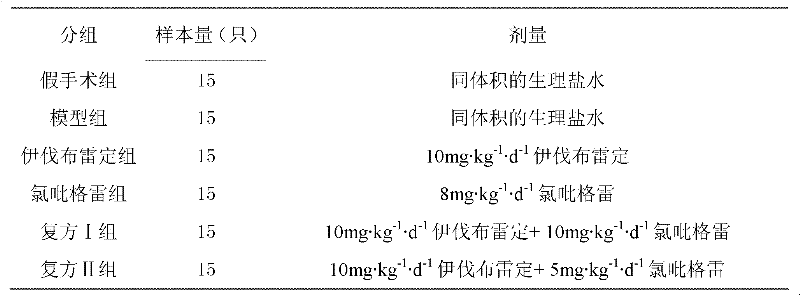
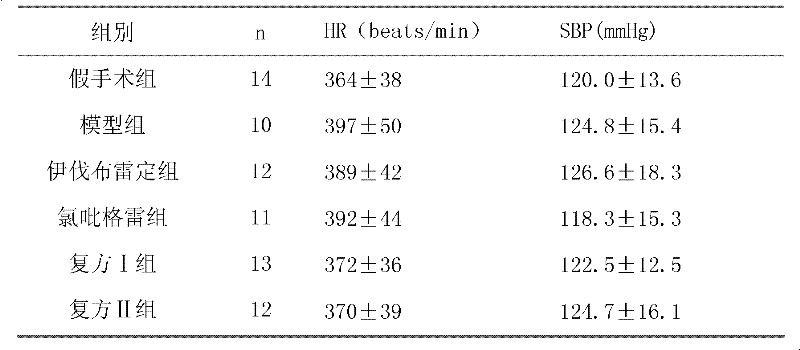
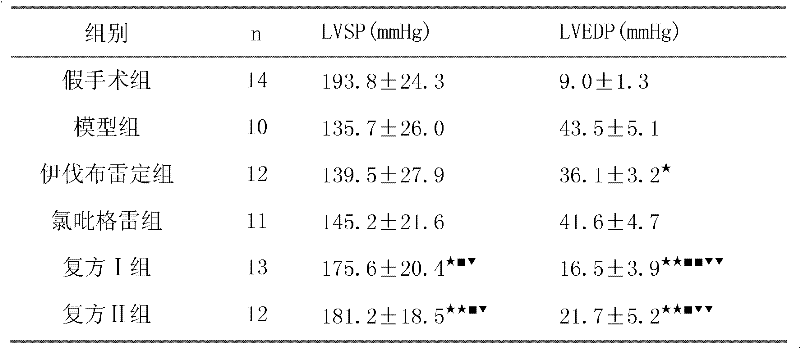
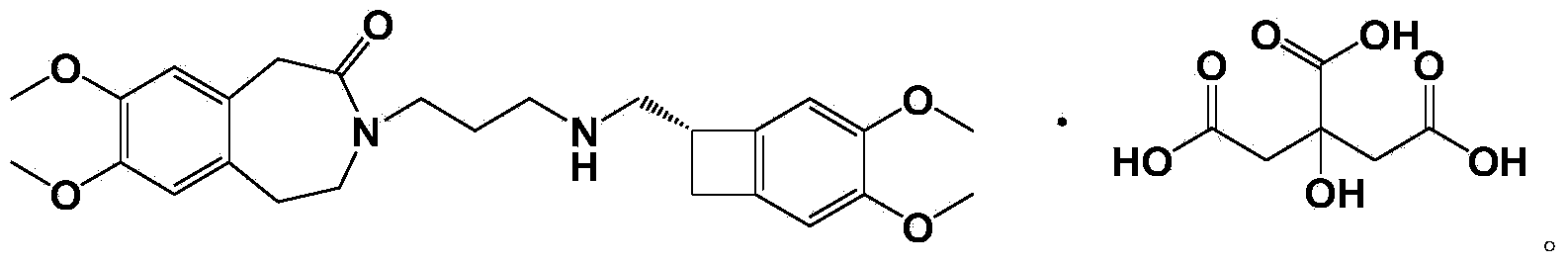
Patents
Literature
93 results about "Ivabradine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
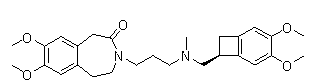
This medication is used by people with a certain heart problem (chronic heart failure), to help prevent it from getting worse and needing treatment in a hospital.
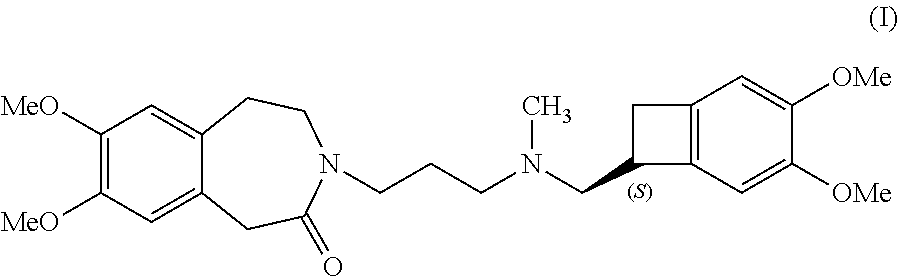
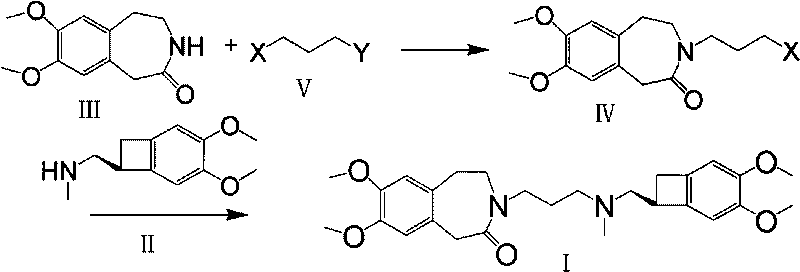
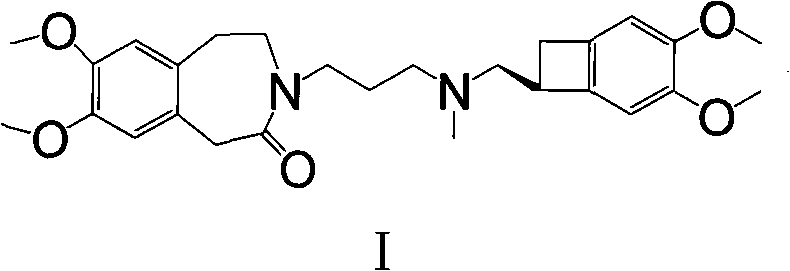
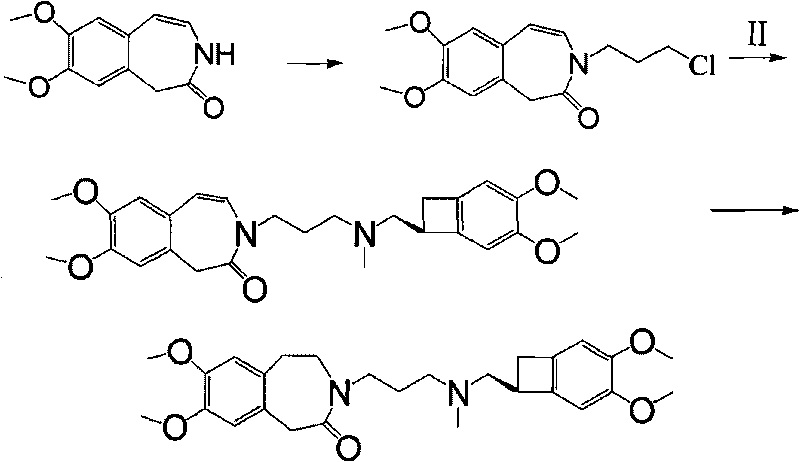
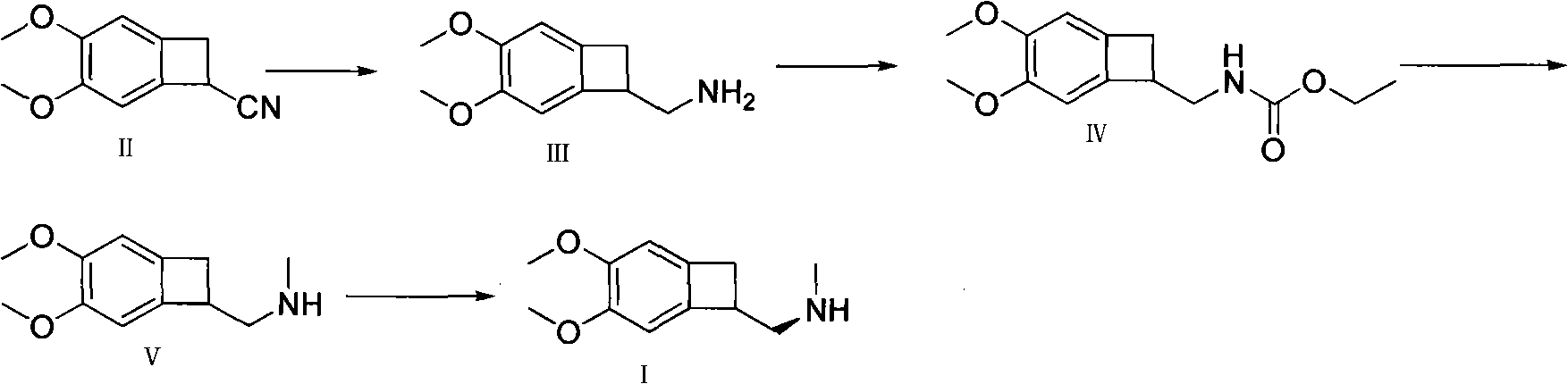
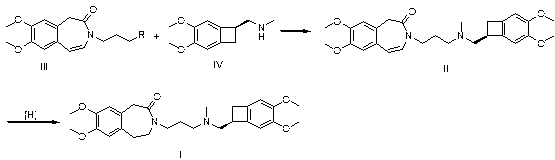
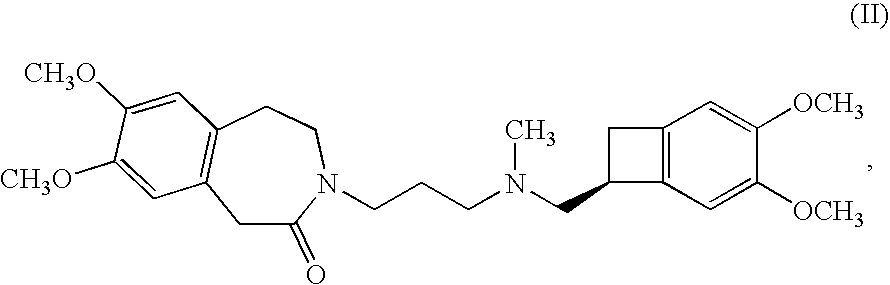
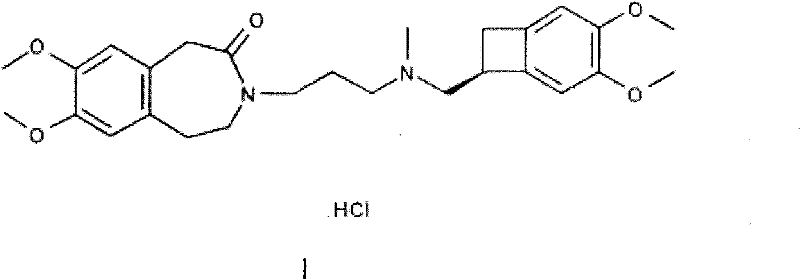
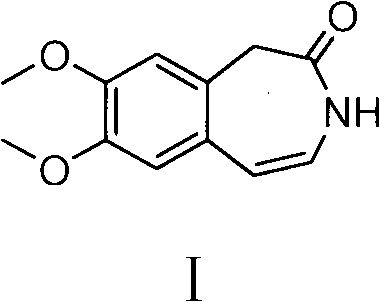
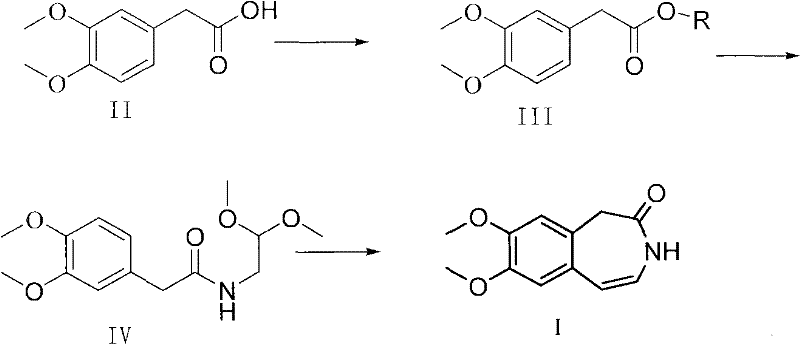
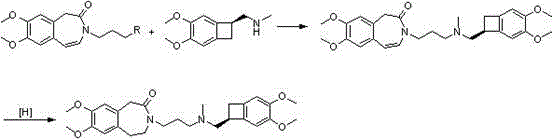
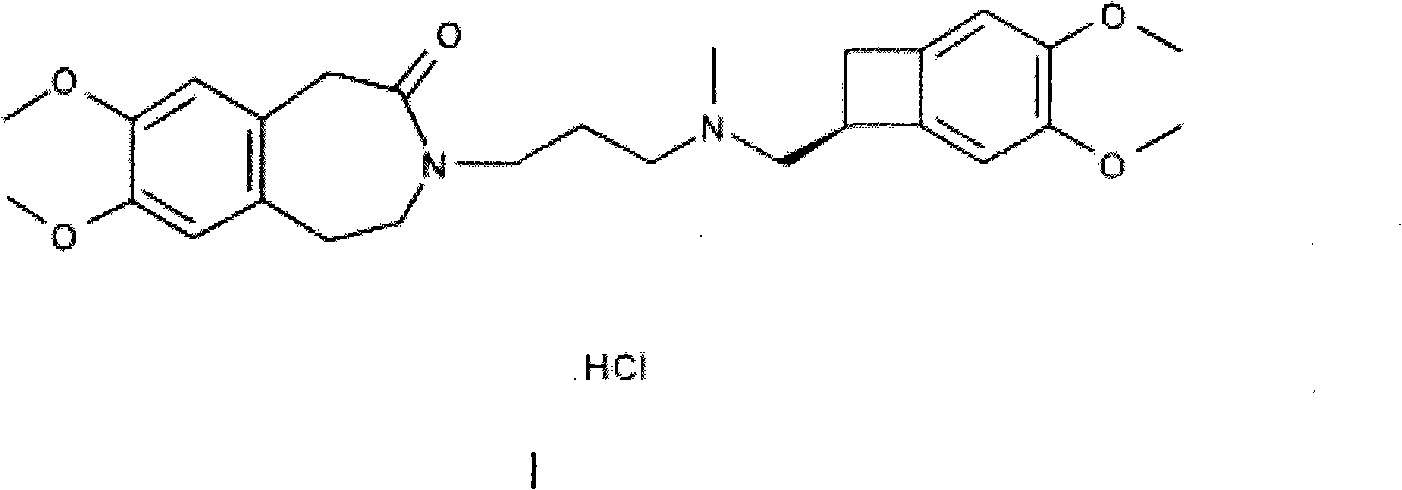
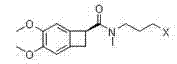
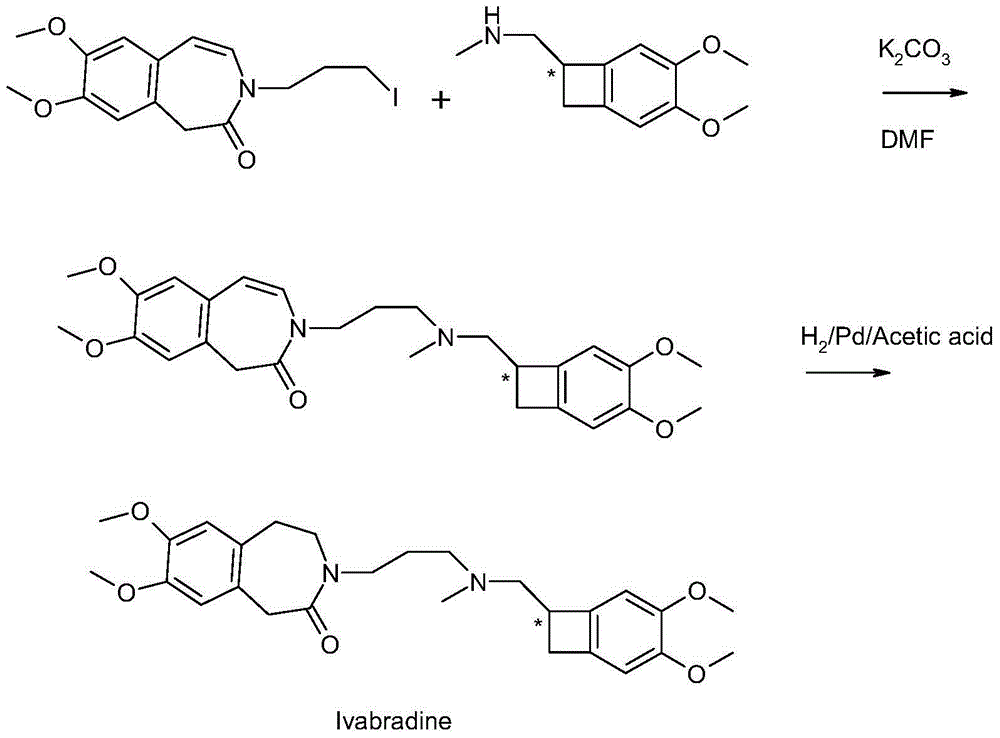
Preparation method of ivabradine
ActiveCN101284813ASimple methodRaw materials are easy to getOrganic chemistryPharmaceutical drugAngina
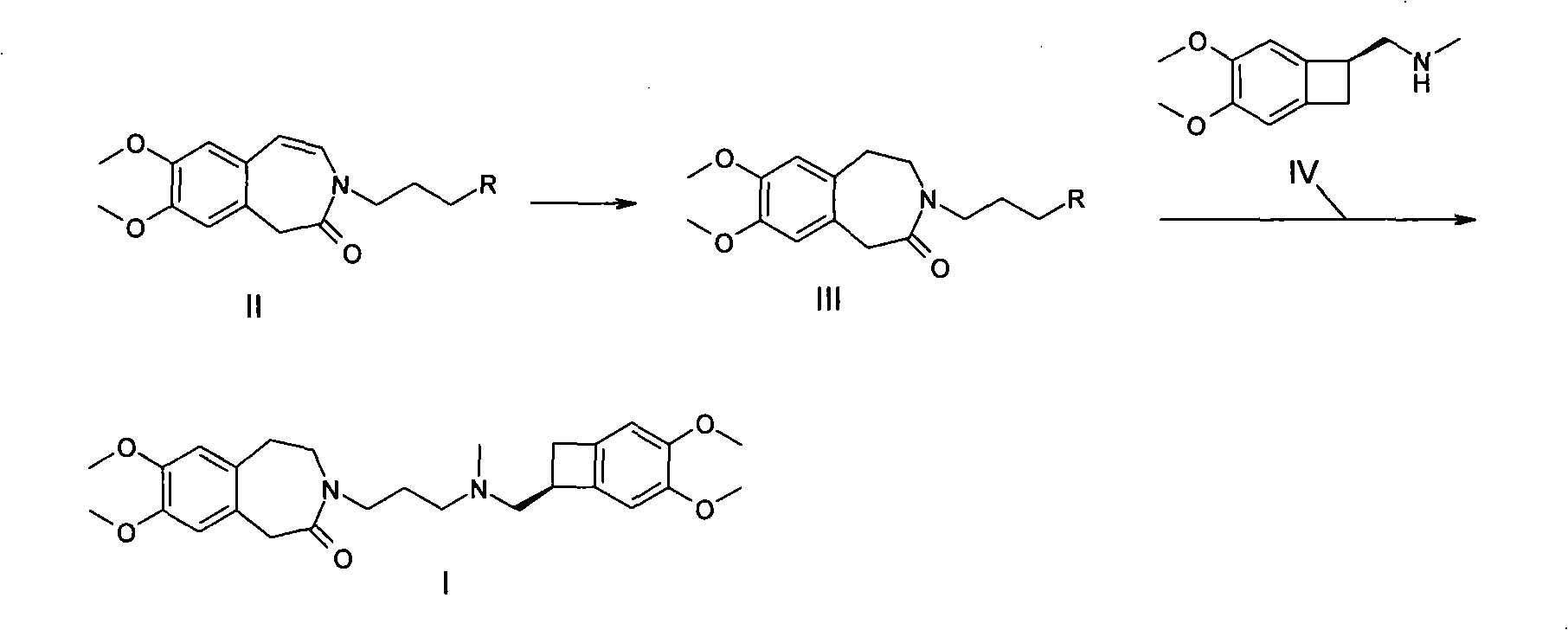
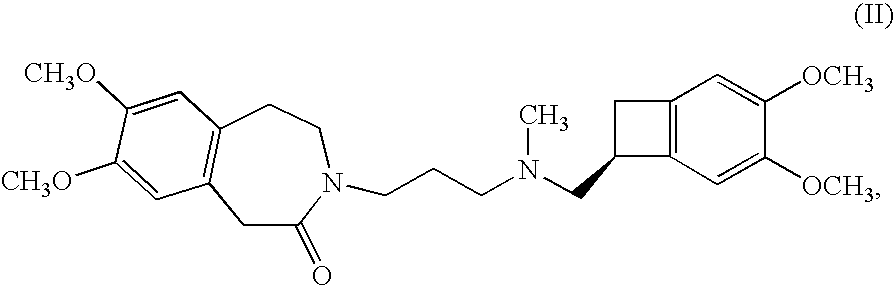
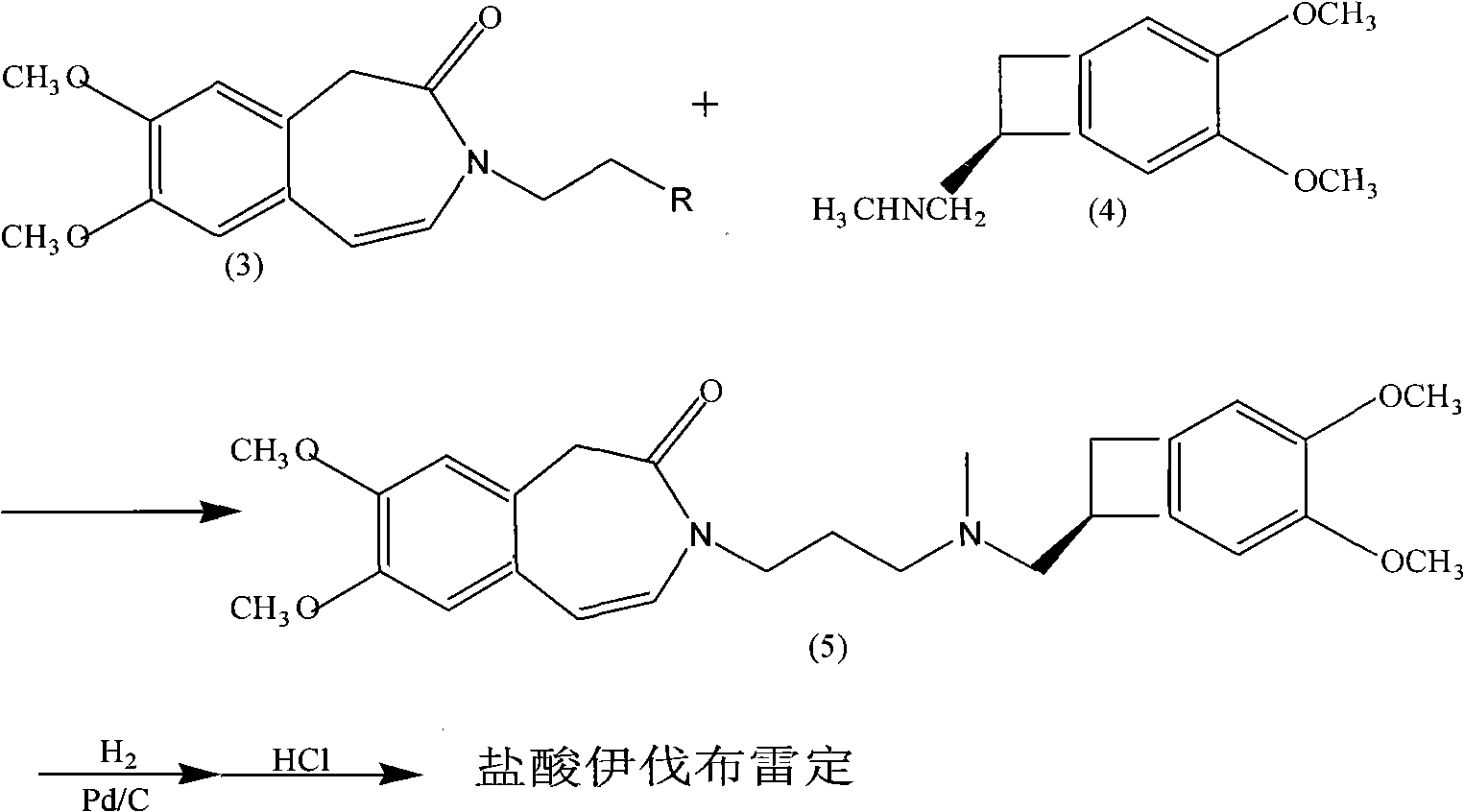
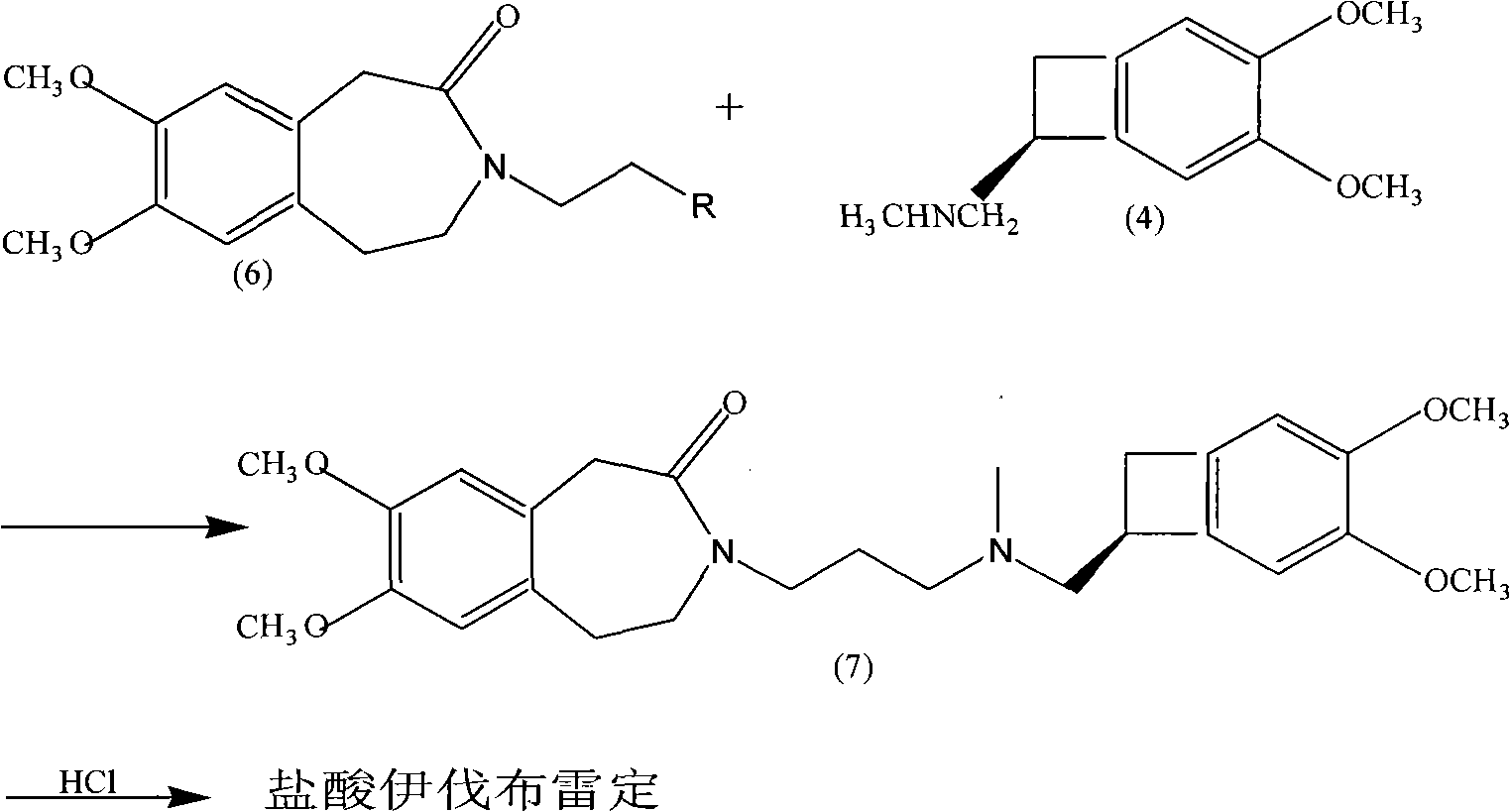
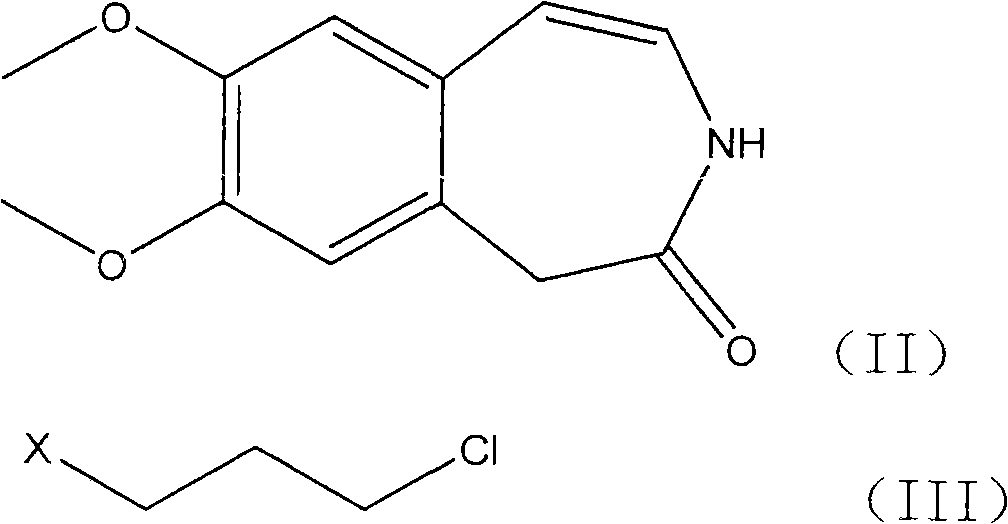
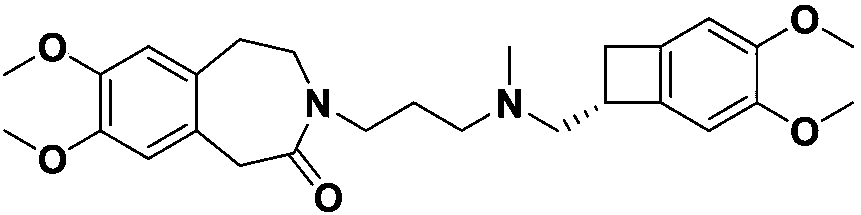
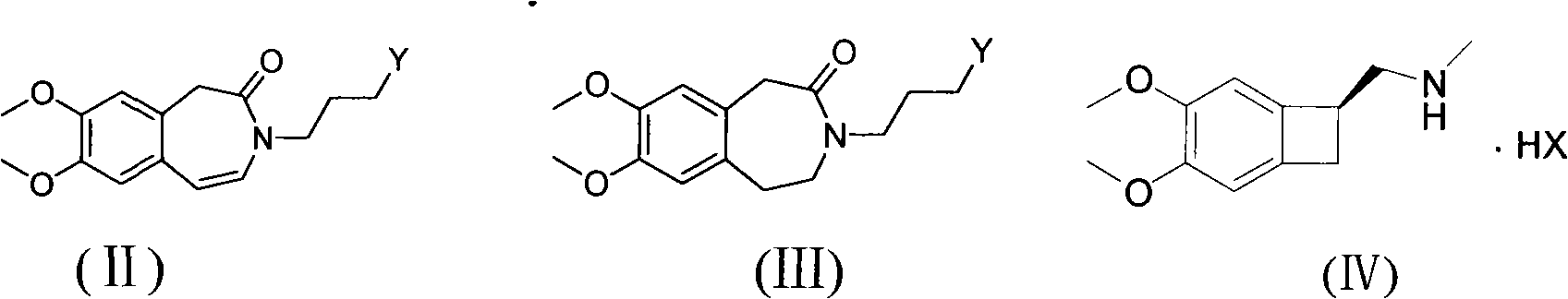
The invention relates to a novel method for preparing angina pectoris medicine Ivabradine (compound I and salt thereof). The method takes a formula II compound as the raw material to obtain a compound III through catalytic hydrogenation, the compound III is made an alkylation reaction with a formula IV compound to produce the compound I. The method is simple and convenient, is easy to obtain raw materials, and is suitable for industrialized production.
Owner:UTOPHARM SHANGHAI +1
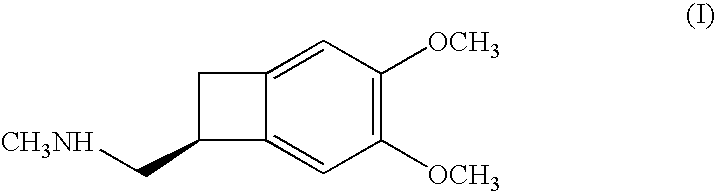
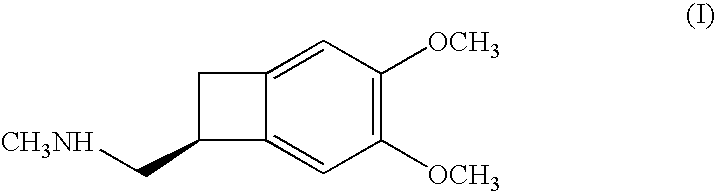
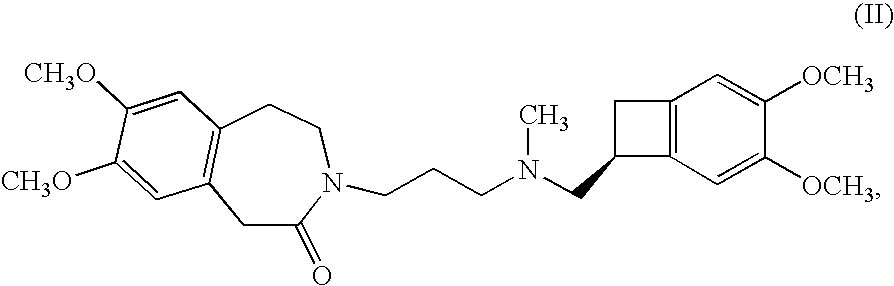
Process for the synthesis of (IS)-4,5-dimethoxy-1-(methylaminomethyl)-benzocyclobutane and addition salts thereof, and to the application thereof in the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid
Process for the synthesis of the compound of formula (I): Application in the synthesis of ivabradine, addition salts thereof with a pharmaceutically acceptable acid, and hydrates thereof.
Owner:LES LAB SERVIER
Ivabradine adsorbates
InactiveUS20170100408A1Efficient administrationPowder deliveryGranular deliveryMedicineSubject matter
The subject-matter of the present invention is a novel, non-salified ivabradine solid form, in particular an ivabradine form adsorbed on an inert carrier. The subject-matter of the invention is also a process for preparing said solid form, its use in therapy and pharmaceutical compositions comprising it.
Owner:LAB CHIM INTERNAZ
Method for synthesizing Ivabradine
InactiveCN101723897AMild conditionsEasy to purifyOrganic chemistryCardiovascular disorderBenzeneKetone
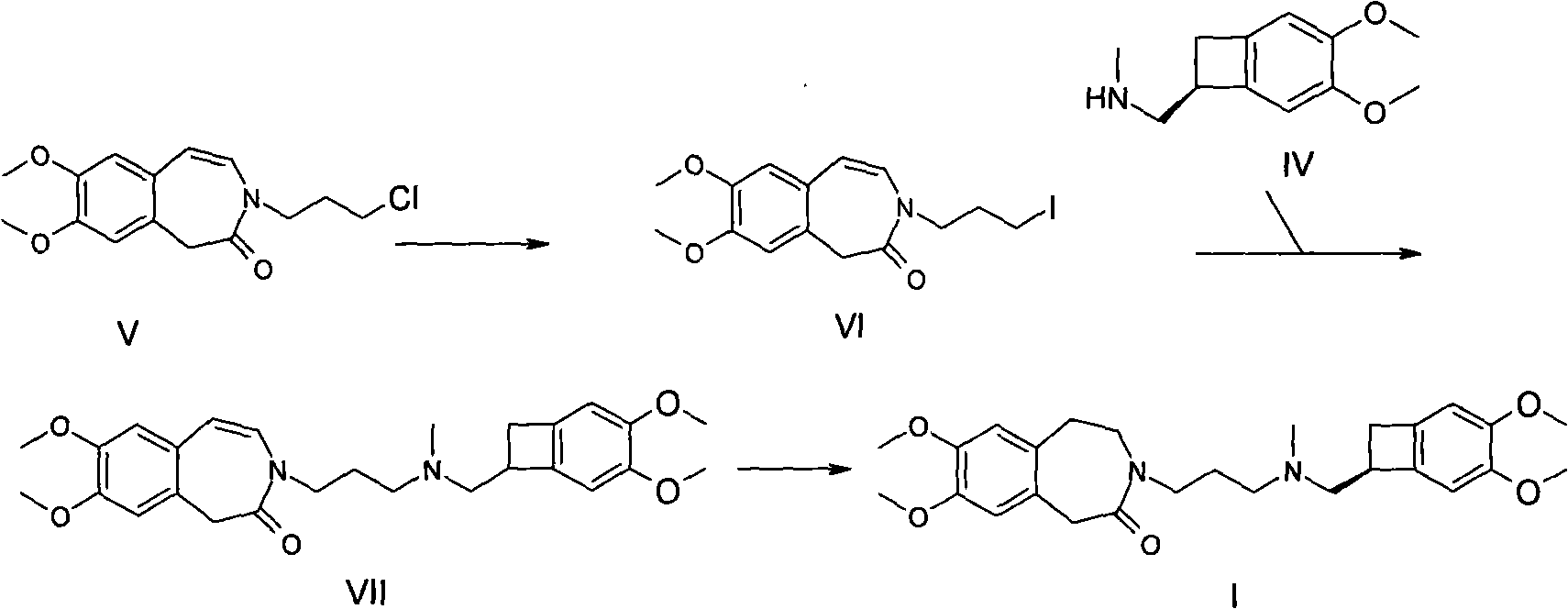
The invention relates to a method for synthesizing Ivabradine or salts thereof, which is prepared by two steps of synthesizing an intermediate N-(3-substitutedpropyl)-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepin-2-ketone by 7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepin-2-ketone, and further reacting with (1S)-4,5-dimethoxy-1-[(methylamino)methyl] benzocyclobutane. By using the method in the invention for preparing the Ivabradine or salts thereof, the reaction post-treatment is simple, the product purity is high, the yield is high, and the method is favor of industrialized production.
Owner:QILU PHARMA
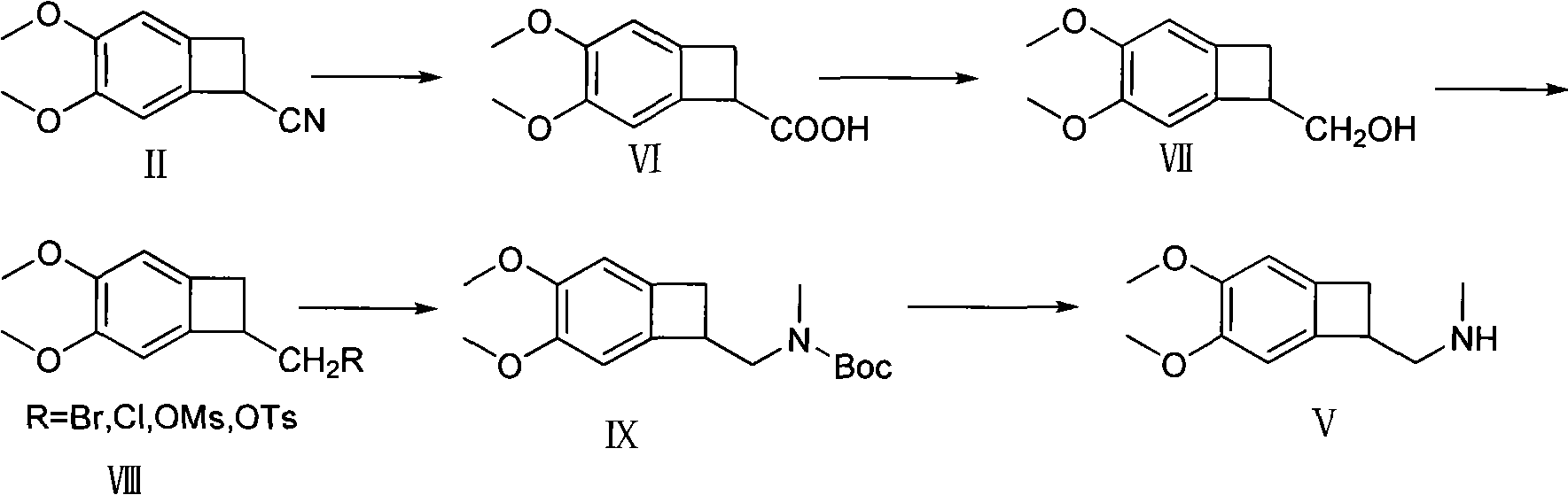
Synthetic method of (1S)-4,5-Dimethoxy-1-(aminomethyl)benzocyclobutane
ActiveCN101857549AHigh purityHigh yieldOrganic compound preparationBulk chemical productionAlkyl transferEnantiomer
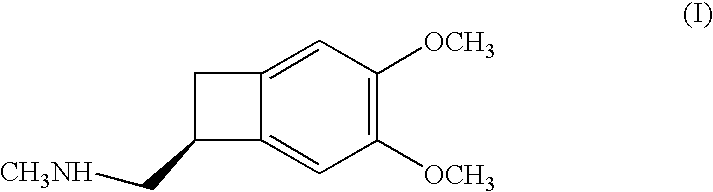
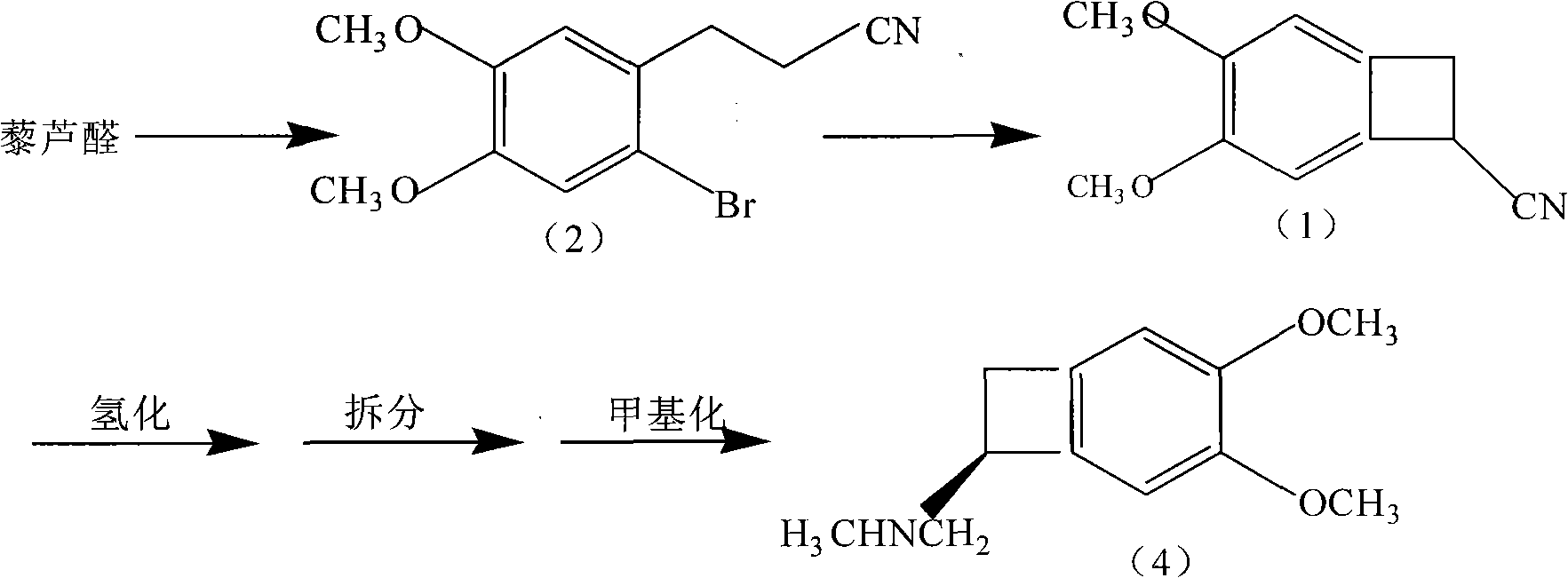
The invention provides a synthetic method of (1S)-4,5-Dimethoxy-1-(aminomethyl)benzocyclobutane, which is an important intermediate of Ivabradine. The method comprises the following steps of: carrying out hydrolyzing, reducing, sulfonylation or halogenation reaction, alkylation and deprotection on 1-cyano-4,5-Dimethoxy-1-cyanobenzocyclobutane used as a raw material to obtain 4,5-Dimethoxy-1-(aminomethyl)benzocyclobutane, and finally splitting with right-hand mandelic acid to obtain the (1S)-4,5-Dimethoxy-1-(aminomethyl)benzocyclobutane. The method has the advantages of high yield, high purity of chemical and enantiomer (more than 99.5 percent), low cost and suitability for industrial production.
Owner:ZHEJIANG MENOVO PHARMA
Novel preparation method for ivabradine
ActiveCN103012268AReduce the difficulty of synthesisEasy to operateOrganic chemistrySynthesis methodsHydrogenation reaction
The invention belongs to the field of pharmaceutical chemical engineering, and relates to a novel synthesis method for ivabradine. The novel synthesis method for ivabradine comprises the following steps of: reacting a compound III with a compound IV in a reaction solvent under the catalysis of an alkali, performing post-treatment to obtain a compound II, and performing hydrogenation reaction under a system containing a catalyst and ammonium formate to obtain a compound I, namely, ivabradine. The method is short in synthesis route, simple to operate, greatly lowered in the difficulty of synthesis for ivabradine, low in cost and high in product yield; and most importantly, the method is good in safety, not involved with high-pressure hydrogenation, free from the use of an inflammable gas, namely, hydrogen, and great in industrialization base and application value.
Owner:江苏宇田医药有限公司
Process for the synthesis of (1S)-4,5-dimethoxy-1-(methylaminomethyl)-benzocyclobutane and addition salts thereof, and to the application thereof in the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid
ActiveUS6982350B2Organic compound preparationOptically-active compound separationIvabradineMedicinal chemistry
Process for the synthesis of the compound of formula (I): Application in the synthesis of ivabradine, addition salts thereof with a pharmaceutically acceptable acid, and hydrates thereof.
Owner:LES LAB SERVIER
Pharmaceutical composition containing ivabradine and ranolazine
ActiveCN101780091BHazard mitigationGood synergyCardiovascular disorderHeterocyclic compound active ingredientsMedicineAngina
The present invention provides a pharmaceutical composition containing active ingredients ivabradine or a pharmacologically acceptable salt thereof and ranolazine or a pharmacologically acceptable salt thereof. Through research, the present invention finds that the combined use of ivabradine and ranolazine effectively alleviates myocardial ischemia, and shows a good synergistic effect on myocardial ischemia diseases such as angina pectoris and coronary heart disease. Therefore, a method for treating myocardial ischemic diseases with better effect and lower adverse reactions has been found, and a good solution has been found for the unsatisfactory treatment effect of myocardial ischemic diseases in clinical practice.
Owner:LUNAN PHARMA GROUP CORPORATION
Process for the synthesis of 1,3,4,5-tetrahydro-2H-3-benzazepin-2-one compounds, and application in the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid
ActiveUS7064200B2Organic chemistry methodsHeterocyclic compound active ingredientsAlkoxy groupPharmaceutical medicine
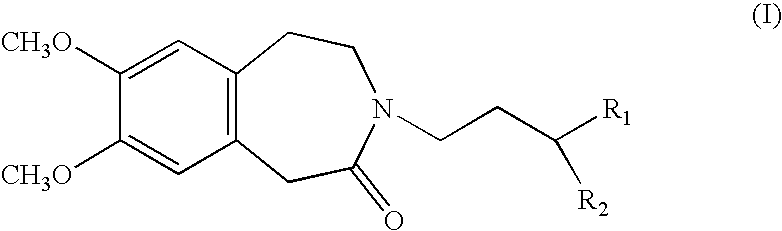
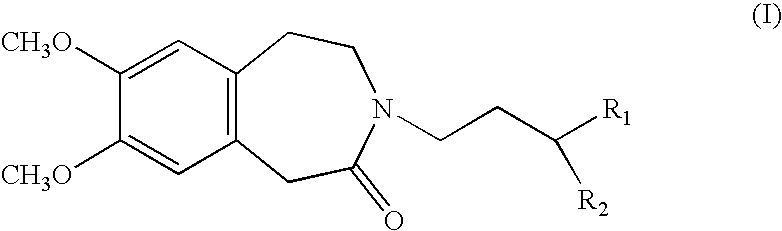
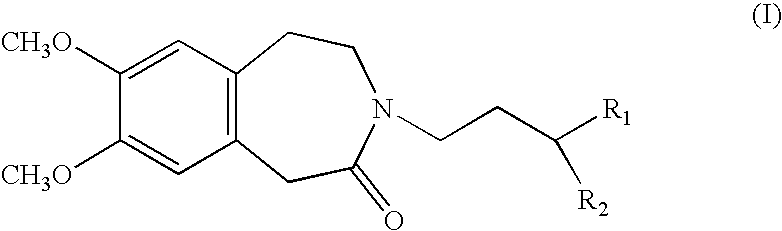
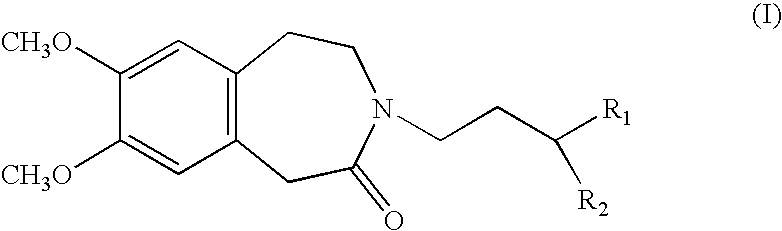
Process for the synthesis of compounds of formula (I):wherein R1 and R2, which may be the same or different, each represent a linear or branched (C1–C8)alkoxy group or form, together with the carbon atom carrying them, a 1,3-dioxane, 1,3-dioxolane or 1,3-dioxepane ring.Application in the synthesis of ivabradine, addition salts thereof with a pharmaceutically acceptable acid, and hydrates thereof.
Owner:LES LAB SERVIER SA
Synthetic method of ivabradine midbody
ActiveCN102464595ACarboxylic acid nitrile preparationOrganic compound preparationOrganic baseBromine
The invention belongs to the technical field of medicines and particularly relates to a synthetic method of an ivabradine midbody, i.e. 4, 5 dimethoxy-1-hydrogen radical-benzocyclobutene; the synthetic method comprises the step of leading compound 3-(2-bromine-4, 5-dimethoxy phenyl) and propionitrile serving as raw materials to be subjected to substitution reaction in a non-protonic solvent to generate the compound 4, 5 dimethoxy-1-hydrogen radical-benzocyclobutene under the combined action of organic base and diethylamine; and the synthetic method is simple, convenient and safe in operation, has higher yield (more than 75%) and purity (more than 99%) and is suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Western medicinal compound for preventing or treating myocardial ischemic chronic heart failure and application thereof
ActiveCN102379877ASignificant progressReduce adverse reactionsHeterocyclic compound active ingredientsCardiovascular disorderWestern medicineActive component
The invention relates to a pharmaceutical composition for preventing or treating patients with myocardial ischemic chronic heart failure and an application thereof. Active components of the composition are composed of ivabradine and clopidogrel or its pharmaceutically acceptable salts, wherein the pharmaceutically acceptable salts are bisulfate, sulfate, camphorsulfonate and hydrochloride. The western medicinal compound provided by the invention can be used to cooperatively treat patients with myocardial ischemic chronic heart failure and simultaneously reduce adverse effects of the medicine.
Owner:南通金羽禽业发展有限公司
Medicinal composition containing silver ester medicine and ibobulodine
Owner:LUNAN PHARMA GROUP CORPORATION
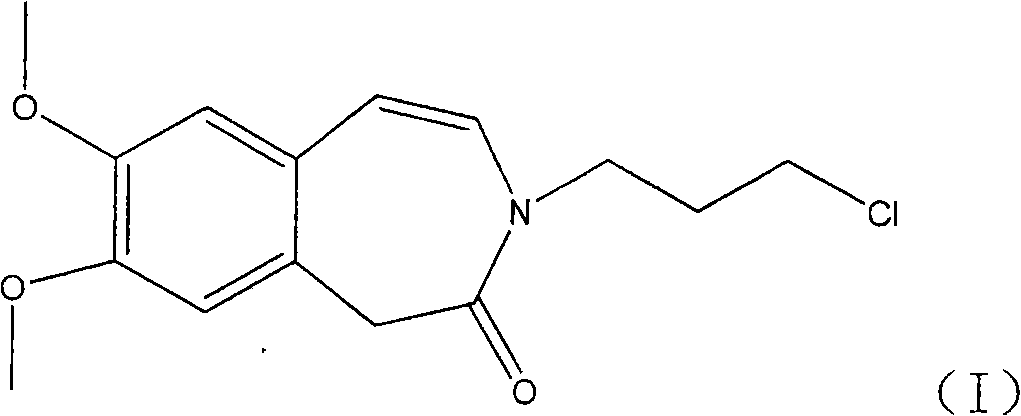
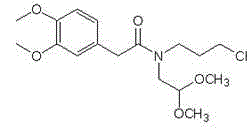
Preparation of 3-(3-chlorine propyl)-7, 8-di methoxy-1, 3-dihydrogen-2H-3-benzo aza -ketone
InactiveCN101343249AEasy to handleSuitable for industrial scale applicationsOrganic chemistryCardiovascular disorderAlkyl transferKetone
The invention relates to the technique field of a preparation method of an intermediate compound of Ivabradine. The preparation method of 3-(3-chloropropyl)-7, 8-dimethoxy-1, 3-dihydro-2H-3-benazepine-2-ketone comprises: making 7, 8-dimethoxy-1, 3-dihydro-2H-3-benazepine-2-ketone and 1, 3-chlorobromide have the alkylation reaction. The preparation method unexpectedly selects an alkaline reagent used commonly, and has advantages of mild reaction conditions, safety and reliability, and rather high yield, and the method fully overcomes the defects of strong water absorption, easy deterioration, or easy explosion under rather low temperature of the alkylation reagent used in the prior art, and is extremely applied to the industrialized big-production.
Owner:SHANGHAI INST OF PHARMA IND
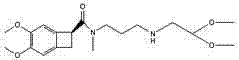
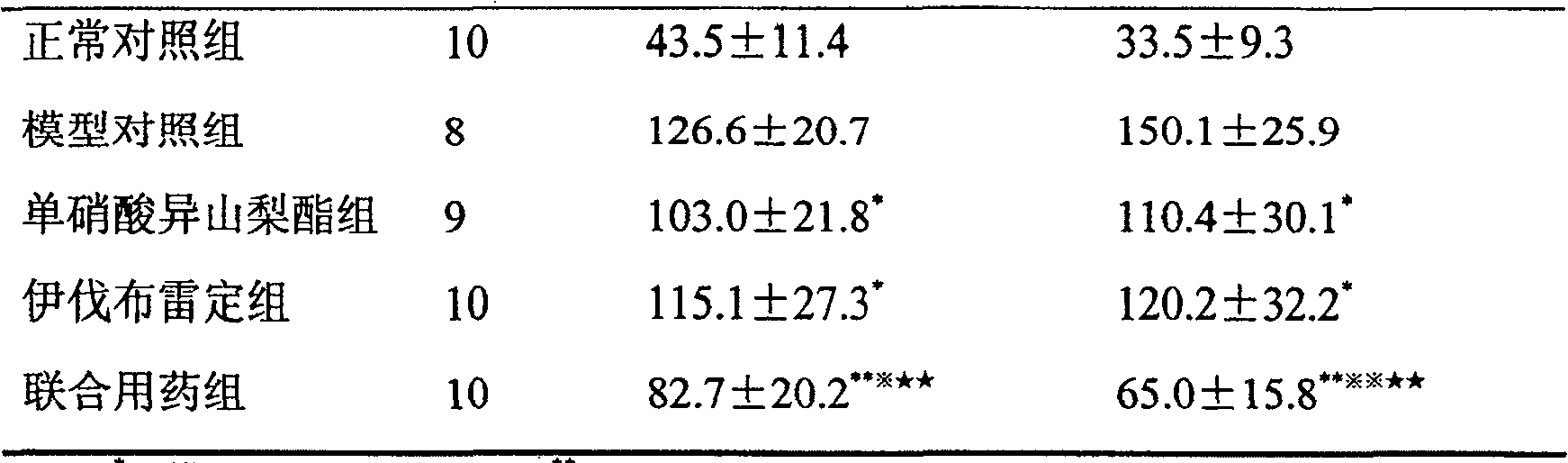
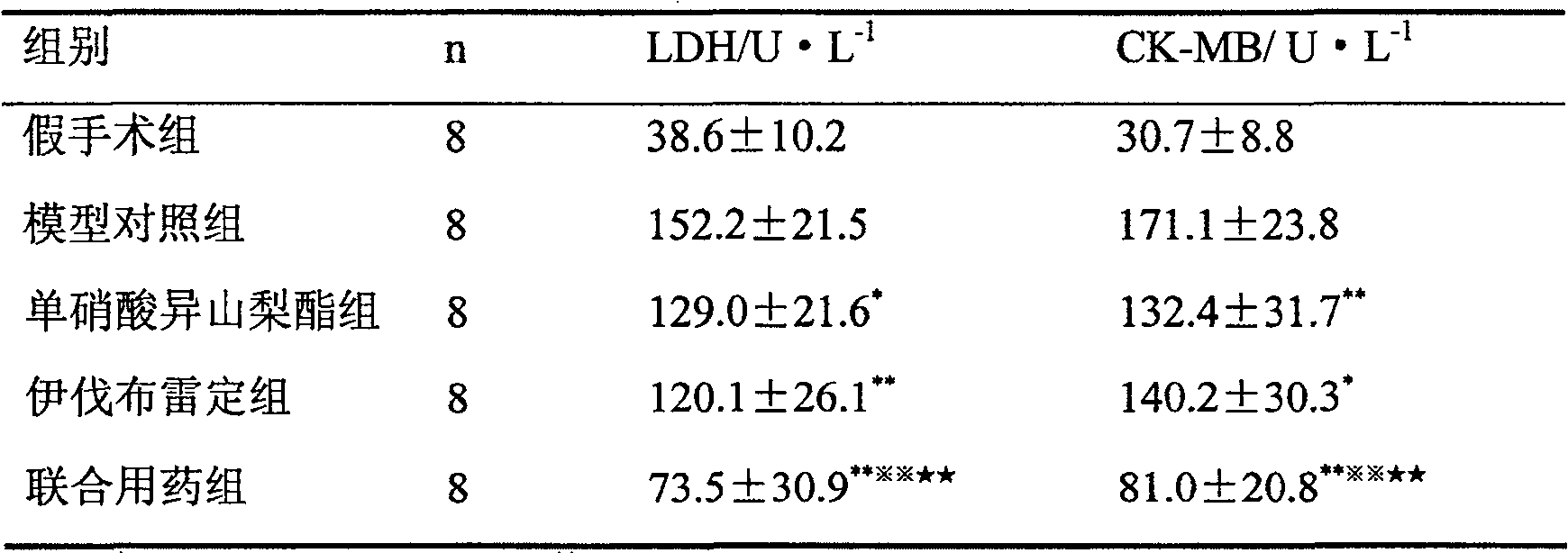
New application of composition containing isosorbide mononitrate and ivabradine
ActiveCN106176761AGood treatment effectImprove diastolic function of the heartCardiovascular disorderHeterocyclic compound active ingredientsSide effectIsosorbide mononitrate
The invention belongs to the field of medicine and particularly relates to an application of a pharmaceutical composition taking isosorbide mononitrate and ivabradine as active pharmaceutical ingredients in preparation of medicine for treating diastolic heart failure. By conformation through a lot of pharmacology experiment, the isosorbide mononitrate and the ivabradine can obviously improving heart diastolic function of patients with diastolic heart failure, and remarkable synergistic effect is achieved in the aspect of treating the diastolic heart failure. The pharmaceutical composition is obvious in treating effect and little in side effects in treating the diastolic heart failure, and is quite good in medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method for ivabradine impurities
InactiveCN108424389AImprove medication safetyEasy to prepareOrganic chemistryBulk chemical productionIvabradineImpurity
The invention discloses a preparation method for ivabradine impurities. According to the preparation method, 3-hydroxy-4-methoxyphenylacetic acid (as shown in a formula 1a) and 4-hydroxy-3-methoxyphenylacetic acid (as shown in a formula 1b) are respectively used as a starting raw material and subjected to multiple steps of reactions to prepare two impurities of ivabradine. The preparation method of the invention is simple and realizes high-purity preparation; and the prepared impurities can be used for qualitative and quantitative analysis so as to improve the medication safety of ivabradine.
Owner:ZHEJIANG JINGXIN PHARMA
Preparation method for Ivabradine
The invention discloses a preparation method for Ivabradine. Catalytic hydrogenation is carried out on a formula (II) compound to obtain a formula (III) compound, reaction is carried out between the formula (III) compound and a formula (IV) compound under the action of base catalysis, and a catalyst is removed through filtration and separated to obtain the target compound.
Owner:BEIJING D VENTUREPHARM TECH DEV
Preparation method of 7,8-dimethoxy-1,3-dihydro-2h-3-benzazepin-2-one
The invention belongs to the field of medicinal preparations and provides a method for preparing a key intermediate of ivabradine, namely 7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one. The method is characterized in that: a form of active ester is obtained by activating a carboxyl group of 3,4-dimethoxyphenylacetic acid under the catalysis of alkali, and then the active ester and 2,2-dimethoxyethylamine are ammoniated and cyclized to form a target product, so that the conditions such as adoption of thionyl chloride, high-temperature distillation and the like which are not suitable for industrialized production are avoided, the yield of the reaction is remarkably improved and reaches 75 to 80 percent, and the method is more suitable for large-scale industrialized production.
Owner:QILU PHARMA CO LTD
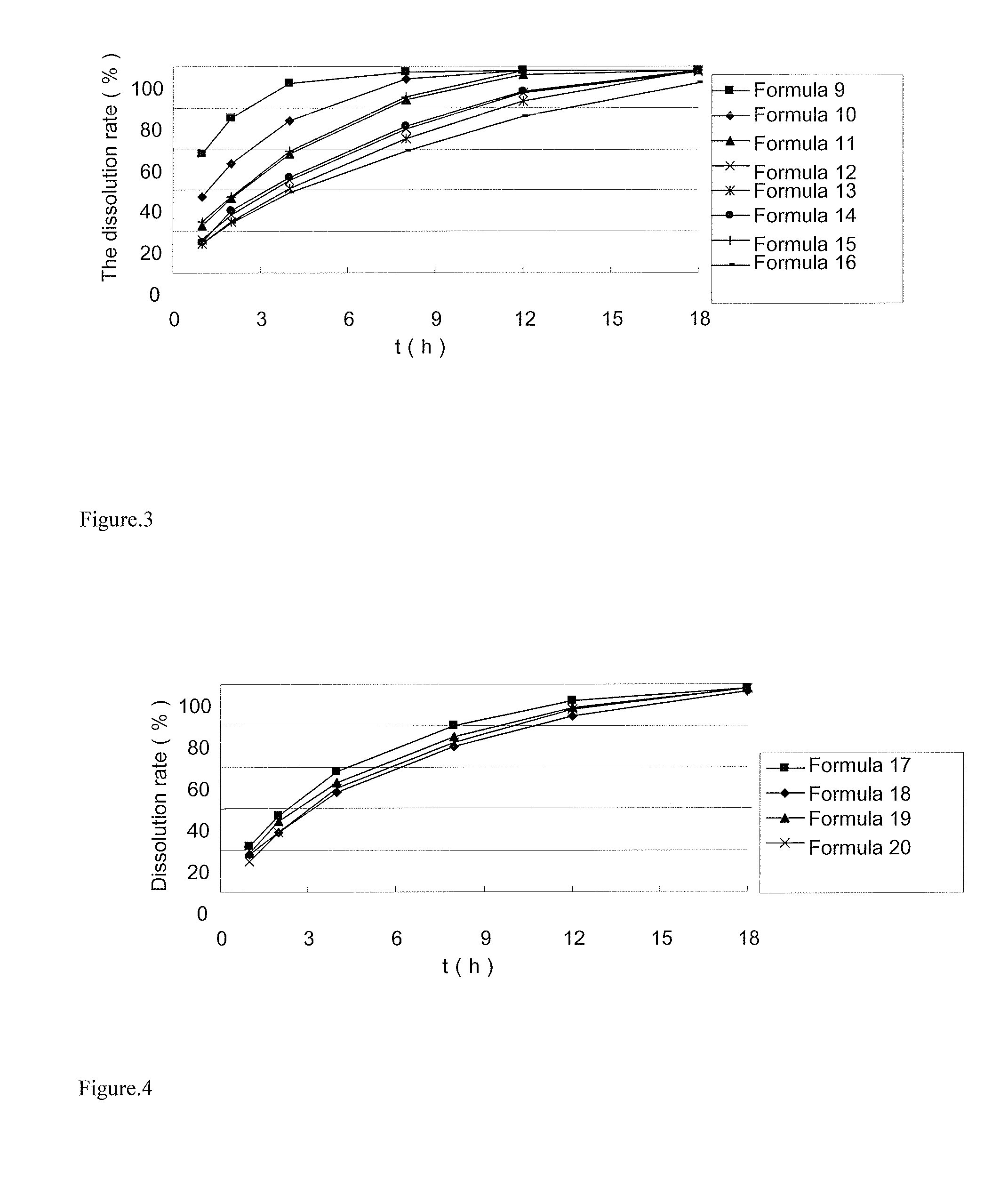
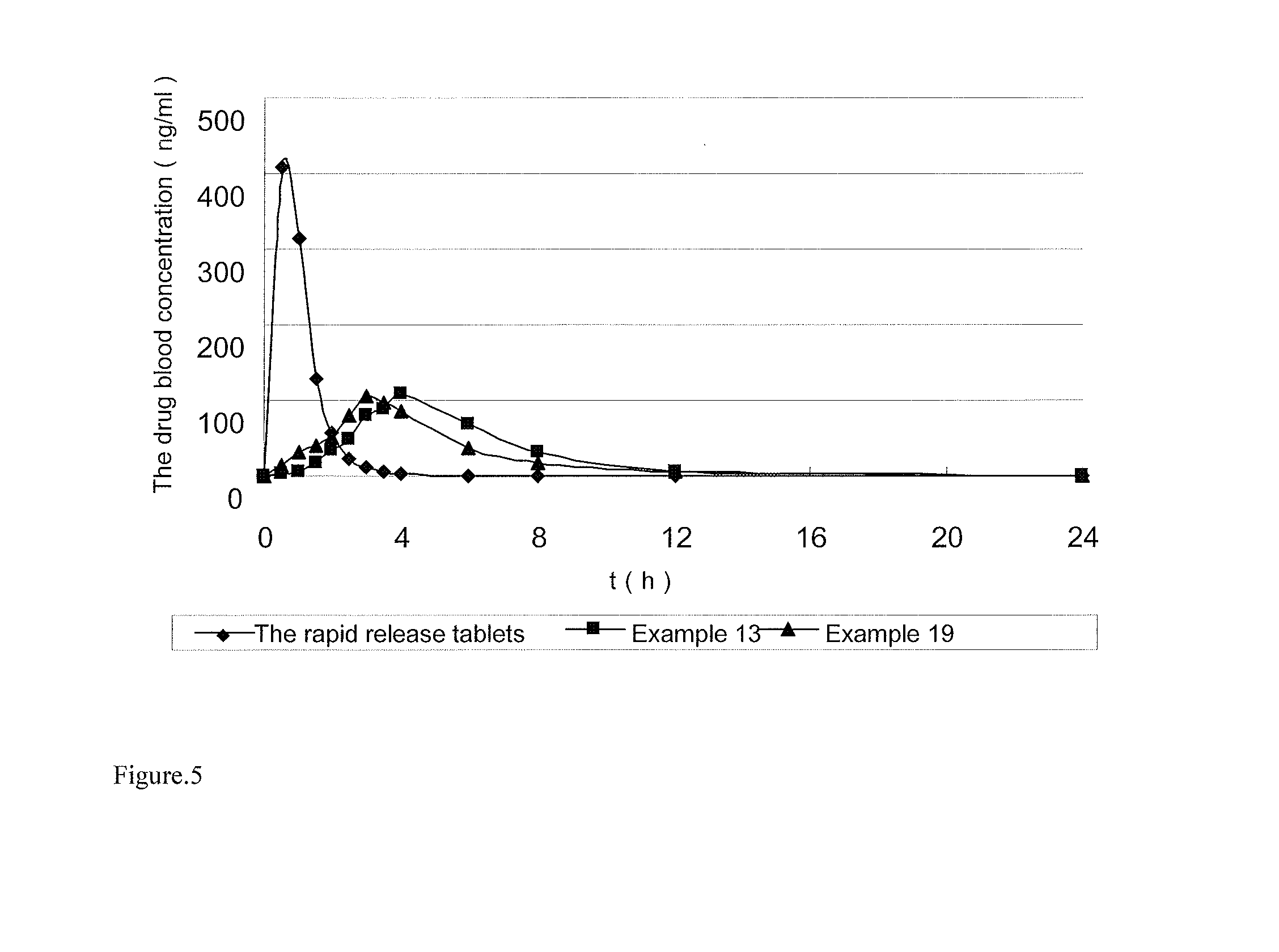
Sustained-release preparation of ivabradine or pharmaceutically acceptable salts thereof
ActiveUS20140179683A1Reduce frequencyGood effectBiocidePharmaceutical non-active ingredientsPolyvinyl acetateIvabradine
Disclosed is a sustained-release preparation of ivabradine or pharmaceutically acceptable salts thereof. The preparation contains ivabradine or pharmaceutically acceptable salts thereof and a sustained-release framework material, wherein the sustained-release framework material is selected from polyoxyethylene, or a mixture of polyoxyethylene and polyvinyl acetate or polyvinyl pyrrolidone.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
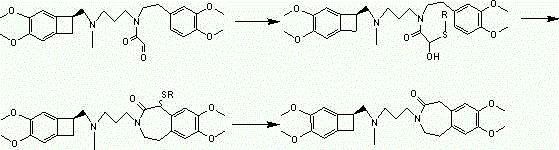
Preparation method for ivabradine and intermediate thereof
ActiveCN104447553AHigh yieldHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsTosylhydrazoneIvabradine
The invention provides a preparation method for ivabradine and an intermediate thereof dehydrogenated ivabradine. The preparation method of the dehydrogenated ivabradine comprises: step a1, enabling a compound shown as a formula II and a compound shown as a formula III to have a nucleophilic substitution reaction in a polar aprotic solvent in the presence of an acid binding agent and a composite phase-transfer catalyst to generate dehydrogenated ivabradine; and step b1, performing separation and purification on dehydrogenated ivabradine obtained in the step a1. The composite phase-transfer catalyst is composed of a quaternary ammonium salt phase-transfer catalyst and a polyether phase-transfer catalyst with the mass ratio of 1-10:1, and X in the formula II is selected from Cl, Br, I, sulfonyloxy, methane sulfonyloxy, benzene sulfonyloxy, p-methylbenzene sulfonyloxy, o-methylbenzene sulfonyloxy or m-methylbenzene sulfonyloxy. The method is capable of substantially shortening the time of nucleophilic substitution reaction, improving product purity, avoiding a column chromatography process and reducing production cost.
Owner:GUANGDONG ZHONGSHENG PHARMA
Set of intermediate compounds used for synthesis of Ivabradine, and applications thereof
ActiveCN104829470ALow priceReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationBiochemical engineeringN acylation
The invention provides a set of intermediate compounds used for synthesis of Ivabradine and a preparation method thereof, and also provides a method used for synthesizing Ivabradine from the intermediate compounds. According to the method, the set of intermediate compounds are subjected to a plurality of N alkylation or N acylation so as to obtain a compound IV; and Ivabradine is directly synthesized from the compound IV. The method is short in synthesis route; raw materials are simple and easily available; reaction sequence is reasonable; operation is simple and high in efficiency; the method is green and friendly to the environment; synthesis difficulty of Ivabradine is reduced greatly; cost is low; product yield is high; and the method is suitable for industrialization production.
Owner:江苏宇田医药有限公司
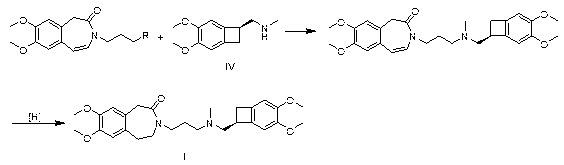
Novel synthetic method of ivabradine and novel intermediate product of ivabradine
ActiveCN102827080AOrganic compound preparationCarboxylic acid amides preparationIvabradinePhotochemistry
The invention relates a novel synthetic method of ivabradine and a novel intermediate product of the ivabradine. In consideration of huge medicinal value of the ivabradine (compound I) and larger difficulty for synthesizing the ivabradine at present, the invention provides a group of novel benzocyclobutane compounds and a method for synthesizing the benzocyclobutane compounds and a method for synthesizing the ivabradine by the benzocyclobutane compounds, that is, a novel synthetic method of the ivabradine. The method provided by the invention is short in synthetic line, simple to operate, simple in preparation method and low in cost, and the synthetic difficulty of the ivabradine is greatly reduced.
Owner:江苏宇田医药有限公司
Medical composition containing ivabradine and ranolazine
ActiveCN101780091AHazard mitigationGood synergyCardiovascular disorderHeterocyclic compound active ingredientsDiseaseCoronary heart disease
The invention provides a medical composition containing ivabradine or pharmacologically acceptable salts and ranolazine or pharmacologically acceptable salts thereof. Proved by research, the medical composition effectively relieves myocardial ischemia and has good synergetic effect on myocardial ischemia diseases, such as angina, coronary heart disease, and the like by the combined use of the ivabradine and the ranolazine. Thus, a method for treating myocardial ischemia diseases with good effect and low adverse reaction is found. The invention finds a good solution for treating myocardial ischemia diseases clinically with poor effect at present.
Owner:LUNAN PHARMA GROUP CORPORATION
Oral preparations of ivabradine or the medical salt thereof
The invention discloses a kind of oral product of ivabradine (Procoralan)or its medical salt, which can be produced into tablet, capsule and granule dosage form, which is characterized in that medical findings comprises filling agent, adhesive agent and lubricating agent, the weight ratio between ivabradine or its medical salt and medical filling agent is 1: 20-40; weight ratio between ivabradine or its medical salt and adhesive agent is 1: 1-2, and ivabradine or its medical salt and lubricating agent is 1: 0.2-0.4. It is showed by pharmacological experiment that the pharmacological action of ivabradine is very good.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Ivabradine preparation or ivabradine medicinal salt solid preparation and preparation method thereof
ActiveCN101897682AAvoid sudden releaseImprove compliancePill deliveryGranular deliveryVisual symptomsSide effect
The invention relates to an ivabradine preparation or an ivabradine medicinal salt solid preparation and a preparation method thereof. The preparation comprises ivabradine or ivabradine medicinal salt nanocapsules and a medicinal auxiliary material, wherein the mass ratio of the ivabradine or the ivabradine medicinal salt to a carrier material is 5-15:1; the carrier material is a composition prepared from xanthan, silk fibroin and aerosil; and the mass ratio of the xanthan to the silk fibroin to the aerosil is 1.5-4.5:1:1. The optimization proportions of the xanthan, the silk fibroin and the aerosil, melting temperature, heat preservation measure and the effects of various factors on the preparation are selected so as to determine an optimized preparation condition and achieve an entrapment rate of over 95 percent; and the preparation has the advantages of preventing medicament burst release, reducing adverse reactions, improving the compliance of patients and overcoming side effects such as dosage dependence, visual symptoms and the like.
Owner:CSPC OUYI PHARM CO LTD
One group of novel benzene cyclobutane compounds and application of novel benzene cyclobutane compounds in chemical synthesis
ActiveCN102827019AEasy to operateReduce the difficulty of synthesisOrganic compound preparationCarboxylic acid amides preparationChemical synthesisCyclobutane
The invention provides one group of novel benzene cyclobutane compounds, a method for preparing the novel benzene cyclobutane compounds and a method for preparing ivabradine from the novel benzene cyclobutane compounds for solving the problems of higher synthesis difficulty in the ivabradine with huge medicinal value at present. The invention provides a preparation method for the ivabradine. The method is short in synthetic path and is simple to operate so that the synthesis difficulty of the ivabradine is greatly reduced; and in addition, the method has the advantages of low cost, high yield of products and favorable industrial base and application value.
Owner:江苏宇田医药有限公司
Medicinal composition containing silver ester medicine and ibobulodine
The present invention proves through a large number of animal experiments that the combined use of ivabradine and nitrate drugs produces remarkable and unexpected effects in the prevention and treatment of myocardial ischemic diseases. Through a large number of animal experiments, we found that the combined use of nitrates and ivabradine had a significant synergistic effect on chronic myocardial ischemia models. Solid preparations prepared together with nitrates and ivabradine, such as tablets, capsules, dispersible tablets, sustained-release tablets, and sustained-release capsules, are dosage forms suitable for clinical use by patients.
Owner:LUNAN PHARMA GROUP CORPORATION
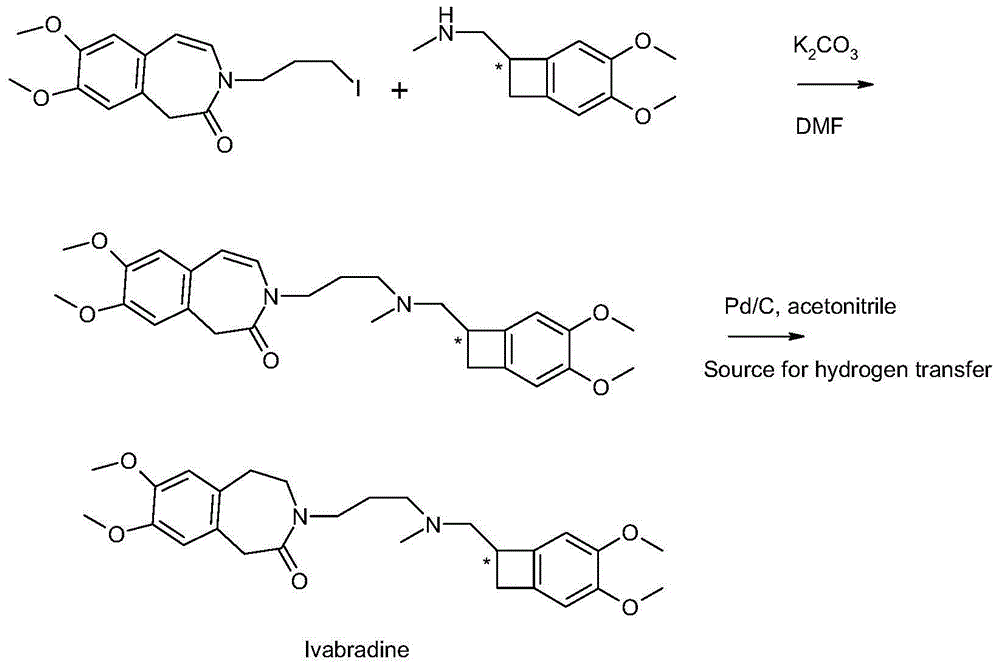
Preparation method for ivabradine and pharmaceutical salt thereof
ActiveCN104788377AHigh yieldAvoid equipment requirements in hydroreductionOrganic chemistryBenzeneHydrogenation reaction
The invention discloses a preparation method for ivabradine. The method includes: in the presence of a noble metal catalyst and a hydrogen transfer reagent, subjecting 3-3{-[{[(7S)-3, 4-dimethoxy benzocyclobutene-7-]methyl}(methyl)amino-propyl]- 7, 8-dimethoxy-1, 3-dihydro-2-hydro-3-benzoazepine-2-one to hydrogen transfer reaction, and after complete reaction, conducting aftertreatment to obtain the ivabradine. The method provided by the invention avoids strict requirements for equipment in hydrogenation reaction, also effectively reduces side effect, and increases the reaction yield.
Owner:ZHEJIANG MENOVO PHARMA
Demethylivabradine salt as well as preparation method and application thereof
ActiveCN103724266AImprove stabilitySimple and complex change is smallPill deliveryCapsule deliverySolubilityHydrobromide
The invention belongs to the technical field of the medicine, and specifically relates to a demethylivabradine salt as well as a preparation method and an application thereof. The demethylivabradine salt is a pharmaceutically acceptable salt, wherein the pharmaceutically acceptable salt includes citrate, hydrobromide, sulfate, phosphate, acetate, trifluoroacetate, lactate, acetonate, malonate, succinate, glutarate, fumarate, maleate, ascorbate, oxalate, mesylate, benzene sulfonate and camphorate, and has excellent stability and solubility, and also has low hygroscopicity and remarkable advantages.
Owner:SHANDONG CHENGCHUANG PHARMA R&D
Process for the synthesis of 1,3,4,5-tetrahydro-2H-3-benzazepin-2-one compounds. and application in the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid
ActiveUS20050228178A1Organic chemistry methodsHeterocyclic compound active ingredientsAlkoxy groupIvabradine
Process for the synthesis of compounds of formula (I): wherein R1 and R2, which may be the same or different, each represent a linear or branched (C1-C8)alkoxy group or form, together with the carbon atom carrying them, a 1,3-dioxane, 1,3-dioxolane or 1,3-dioxepane ring. Application in the synthesis of ivabradine, addition salts thereof with a pharmaceutically acceptable acid, and hydrates thereof.
Owner:LES LAB SERVIER
Enzymatic synthesis of ivabradine midbody and application in the synthesis of ivabradine and addition salts thereof
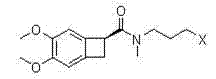
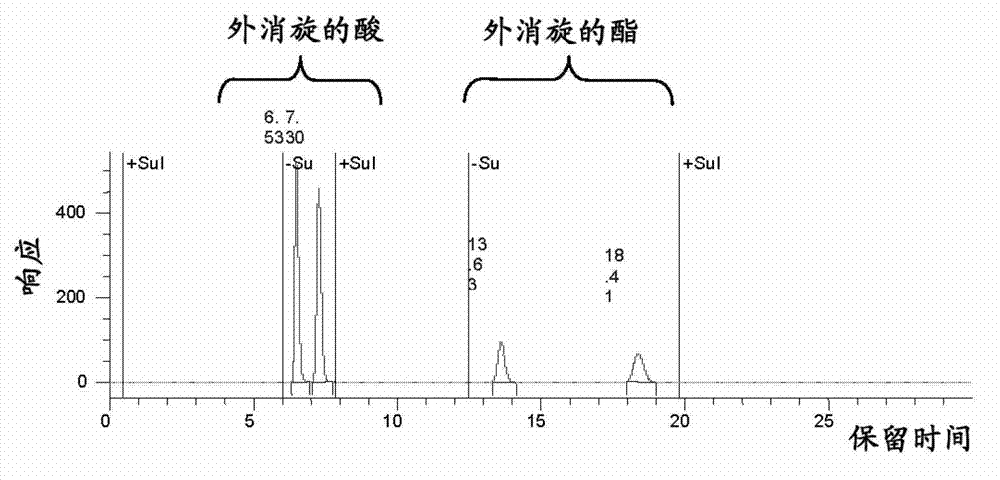
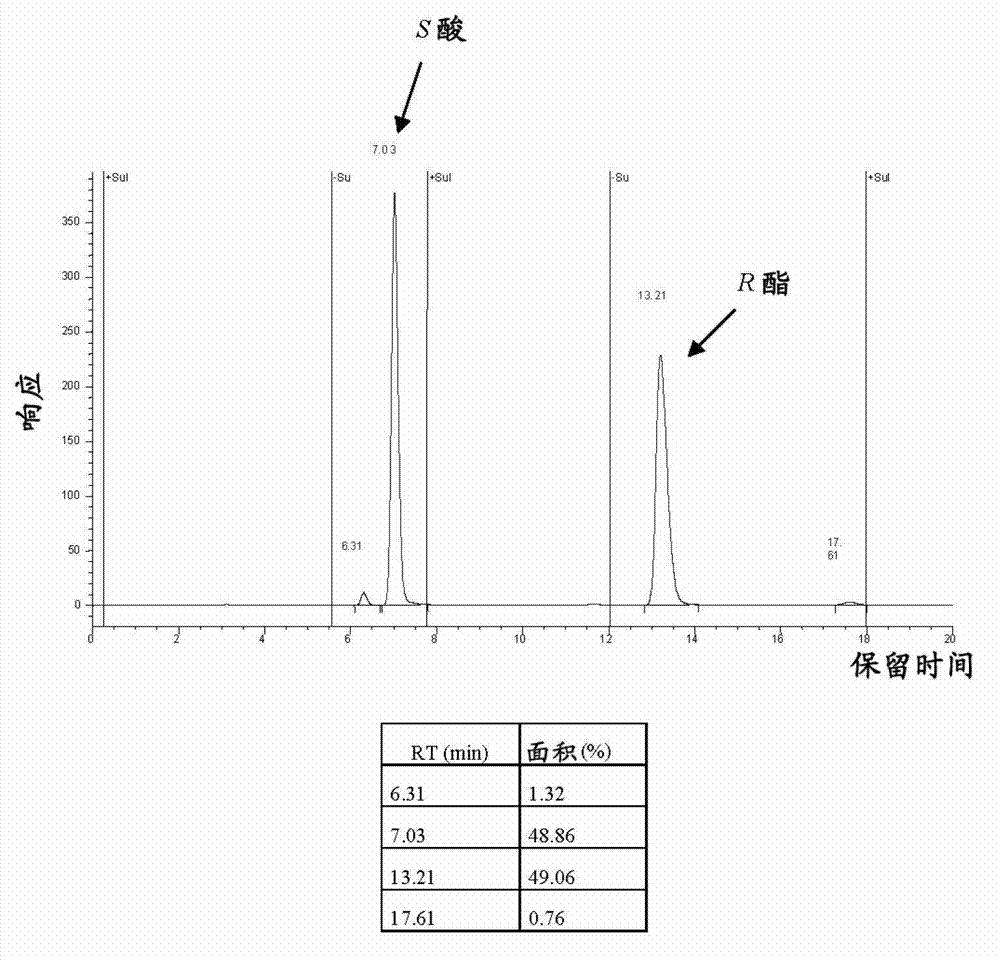
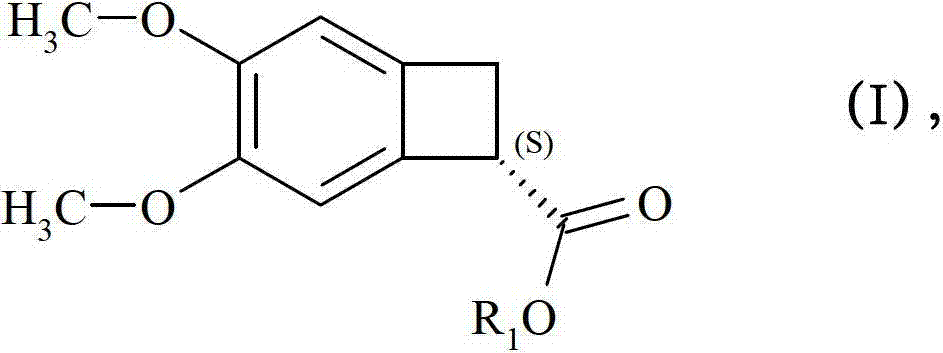
Preparing an optically pure (7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid compound (I), comprises enantioselective enzymatically esterifying a racemic acid or 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid (a), using a lipase or esterase, in a mixture of an alcohol and an organic cosolvent, at a temperature of 25-40[deg] C, where (a) is not pure, has a concentration of 5-500 g / l, and has an enzyme / substrate (E / S) ratio of 10 / 1:1 / 100. Preparing an optically pure (7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid compound (I), comprises enantioselective enzymatically esterifying a racemic acid or 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid (a), using a lipase or esterase, in a mixture of an alcohol of formula (ROH) and an organic cosolvent, at a temperature of 25-40[deg] C, where (a) is not pure, has a concentration of 5-500 g / l, and has an enzyme / substrate (E / S) ratio of 10 / 1:1 / 100. R : linear or branched 1-6C-alkyl, preferably methyl. Independent claims are included for: (1) process-II for preparing an optically pure compound of formula (S)3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid compound of formula (II) by enantioselective enzymatic hydrolysis of the racemic ester or ester compound of formula (b) using the lipase or esterase, in water and in a buffer solution of pH 5-8 or in a mixture of organic solvent and water or buffer at a temperature of 25-40[deg] C, where (b) is not pure, and has E / S ratio of 10 / 1:1 / 100; and (2) process-III for preparing ((S)-3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-trien-7-ylmethyl)-methyl-amine compound (III) comprising either hydrolyzing 3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile (IV) to form (a), enzymatically esterifying (a) to form (I), converting (I) into an optically pure (S)-3,4-dimethoxy-bicyclo[4.2.0]octa-1,3,5-triene-7-carboxylic acid methylamide amide (c) and reducing (c), or hydrolyzing (IV) to form (a), alkylating (a) to form (b), enzymatically hydrolyzing (b) to obtain (II), converting (II) into (c), and reducing (c). [Image].
Owner:SERVIER LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com