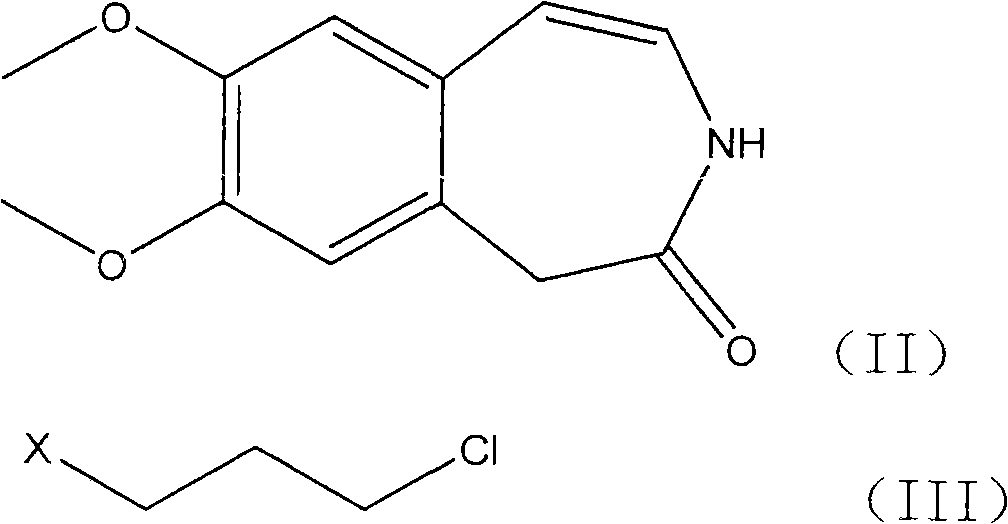
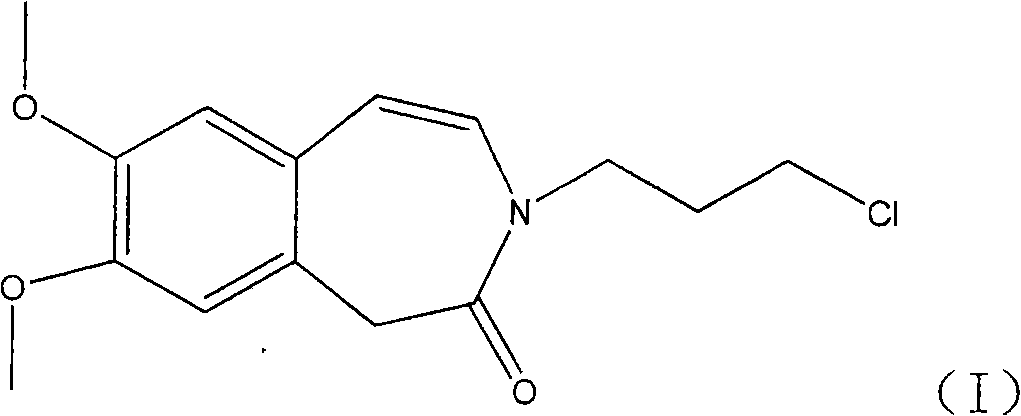
Preparation of 3-(3-chlorine propyl)-7, 8-di methoxy-1, 3-dihydrogen-2H-3-benzo aza -ketone
A technology of dimethoxy and chloropropyl, applied in 3-(3-chloropropyl)-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepine -The field of preparation of 2-ketones can solve the problems of difficult storage, high water absorption, easy explosion and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1.5g (6.8mmol) 7,8-dimethoxy-1,3-dihydro-2H-3-benzazepine -2-Kone, 1.5g (9.5mmol) of 1,3-bromochloropropane and 6ml of DMF were placed in a flask, the temperature of the reaction mixture was raised to 40°C, and 0.8g of 40% sodium hydroxide (8mmol) solution was added. The mixture was then heated to 60°C and stirred for 2 hours; 30 ml of ice water was added and stirred for 1 hour under cooling in an ice-water bath. The solid precipitate was filtered off, washed and dried. The yield of the title compound was obtained in 85%.
Embodiment 2
[0027] 1.5g of 7,8-dimethoxy-1,3-dihydro-2H-3-benzazepine -2-Kone, 1.5g of 1,3-bromochloropropane and 5ml of N-methylpyrrolidone were placed in a flask, the temperature of the reaction mixture was raised to 40°C, and 0.8g of 40% sodium hydroxide solution was added. The mixture was then heated to 60°C and stirred for 2 hours; 30 ml of ice water was added and stirred for 1 hour under cooling in an ice-water bath. The solid precipitate was filtered off, washed and dried. The yield of the title compound was obtained in 83%.
[0028] Synthesis of 3-(3-chloropropyl)-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepine in the presence of potassium carbonate -2-one
Embodiment 3
[0030] 1.5g (6.8mmol) 7,8-dimethoxy-1,3-dihydro-2H-3-benzazepine -2-Kone, 2.7g (19.5mmol) of potassium carbonate and 8ml of DMF were mixed, stirred at 80°C for 1 hour, 1.9g (12.2mmol) of 1,3-bromochloropropane was added at the same temperature, and stirred for 2 hours. Add 50ml of ice water, extract with dichloromethane, concentrate the obtained oil, add appropriate amount of isopropyl ether and ethyl acetate, stir overnight, and filter to obtain the title compound with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com