Method for synthesizing Ivabradine
A synthetic route and compound technology, applied in the fields of drug combination, cardiovascular system diseases, organic chemistry, etc., can solve the problems of chlorinated alkanes prone to side reactions, unreasonable reaction sequence, and cannot be placed for a long time, and achieves easy purification and production. High rate and convenient post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
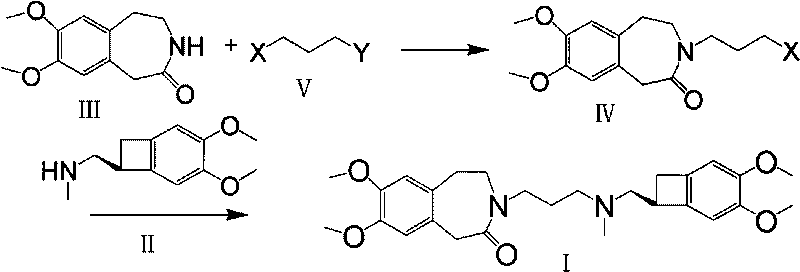
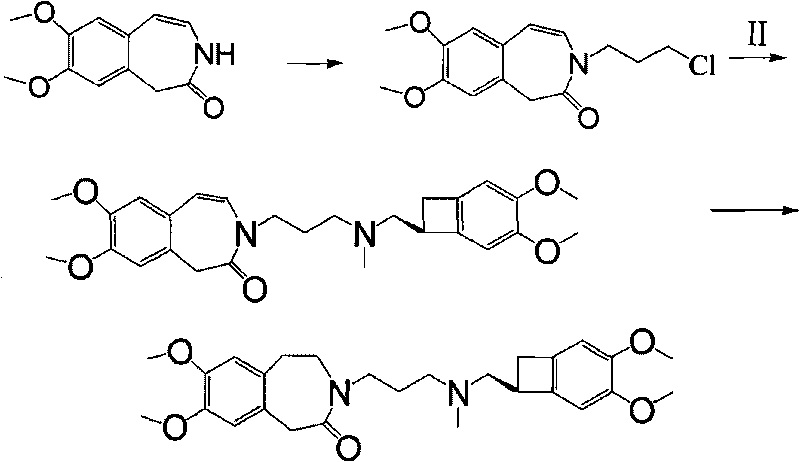
[0041] 1. In 20mlDMSO, add 2.21g (10mmol) of 7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepin-2-one (III) at room temperature Stir to dissolve, add potassium tert-butoxide 1.2g (10.3mmol) in batches, stir at room temperature for 30min, add in batches 1.7g (10.8mmol) of 1-bromo-3-chloropropane in 20ml MDSO solution, react at room temperature for 2h, use Extract with 200ml of water and 200ml of dichloromethane, separate and dry. Distilled under reduced pressure to obtain 2.47 g of oil (IV), with a yield of 83%.
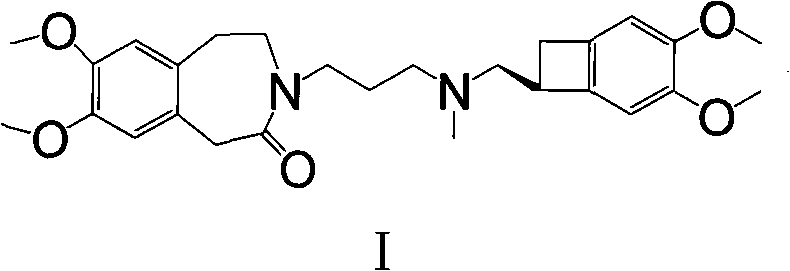
[0042] 2. In 20ml of acetone, add N-(3-chloropropyl)-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine-2 -Kone 2.47g (8.3mol), (1S)-4,5-dimethoxy-1-(methylaminomethyl) benzocyclobutane (II) 1.72g (8.3mol), sodium iodide 1.26 g (15mmolmol), potassium carbonate 2.76g, heated to reflux for 24h, cooled, filtered with suction, and the filtrate was evaporated to dryness under reduced pressure. After separation by column chromatography, 3.38 g of off-white solid (I) was obt...
Embodiment 2
[0044] 1. Intermediate N-(3-chloropropyl)-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepin-2-one 3g ( 10mmol) was added to 30ml of acetone, 1.26g (15mmolmol) of sodium iodide, heated to reflux for 48h, and filtered with hot suction. The filtrate was distilled under reduced pressure to obtain N-(3-iodopropyl)-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine-2-one (IV) 3.78g, yield 97%.
[0045] 2. In 25ml of acetone, add N-(3-iodopropyl)-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine-2 -Kone 3.4g (8.7mmol), (1S)-4,5-dimethoxy-1-(methylaminomethyl) benzocyclobutane formula (II) 1.8g (8.7mmol), potassium carbonate 2.5 g, heated to reflux for 18h, cooled, filtered with suction, and the filtrate was evaporated to dryness under reduced pressure. Purified by column chromatography to obtain 3.38 g of off-white solid (I), with a yield of 83%.
Embodiment 3
[0047] In 25ml of acetone, add N-(3-iodopropyl)-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepin-2-one 3.89g (10mmol), (1S)-4,5-dimethoxy-1-(methylaminomethyl)benzocyclobutane (II) hydrochloride 2.41g (9.9mol), sodium carbonate 4.14g , Heated to reflux for 24h, cooled, filtered with suction, and the filtrate was evaporated to dryness under reduced pressure. Separated by column chromatography, 3.71 g of off-white solid (I) was obtained with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com