Enzymatic synthesis of ivabradine midbody and application in the synthesis of ivabradine and addition salts thereof
A synthetic method and enzymatic technology, which can be used in organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., and can solve problems such as low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Example 1 :3,4-Dimethoxybicyclo[4.2.0]oct-1,3,5-triene-7-carboxylic acid
[0125] 3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carbonitrile (11 g, 58.1 mmol) was suspended in 1N sodium hydroxide solution (70 mL) and The reaction mixture was refluxed (110°C) for 2 hours.
[0126] After returning to ambient temperature, the mixture was acidified with concentrated hydrochloric acid. Precipitation was observed.
[0127] The product was dissolved in 200 mL of dichloromethane, and the aqueous phase was extracted. with MgSO 4 Drying and evaporation gave the title product in 95.9% yield (11.6 g).
Embodiment 2
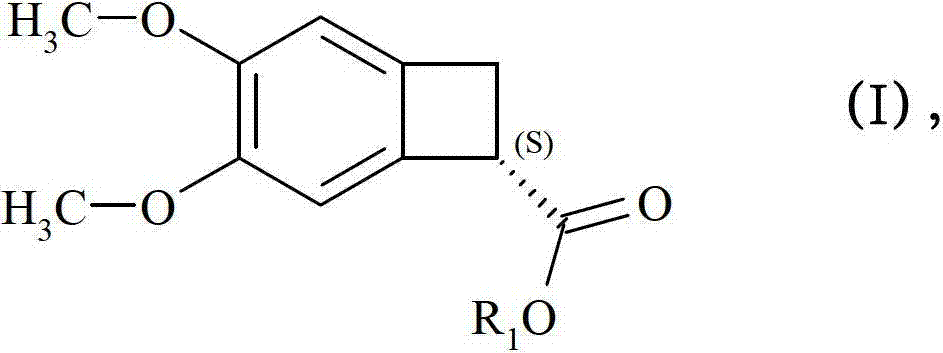
[0128] Example 2 :(7S)-3,4-Dimethoxybicyclo[4.2.0]oct-1,3,5-triene-7-carboxylic acid
[0129] 0.5 g (c=200 g / L) of the racemic acid obtained in Example 1 was dissolved in 2.5 mL of an 8 / 2 mixture of acetonitrile / methanol.
[0130] Then 0.1g (c=40g / L) lipase of Candida antarctica (Candida antarctica) (Novozymes Denmark) was added to the mixture (E / S ratio 1 / 5). The reaction mixture was kept at 30° C. with rotary stirring at 220 rpm for 48 hours.
[0131] The reaction was monitored by chiral phase HPLC under conditions enabling the determination of the enantiomeric excess of both the ester and the acid:
[0132] IC250*4.6 column
[0133] 30% anhydrous ethanol + 0.1% TFA + 70% heptane + 0.1% TFA
[0134] 1ml / min, 25°C, 288nm
[0135]
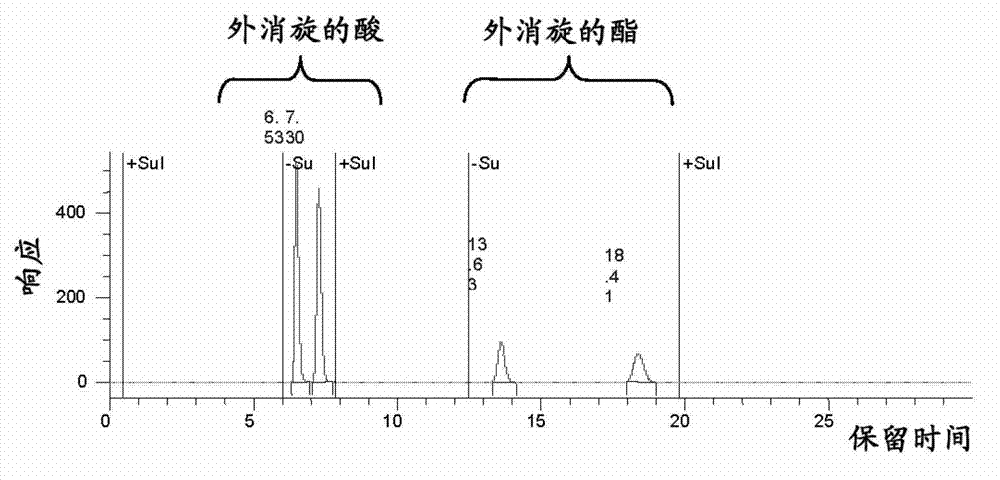
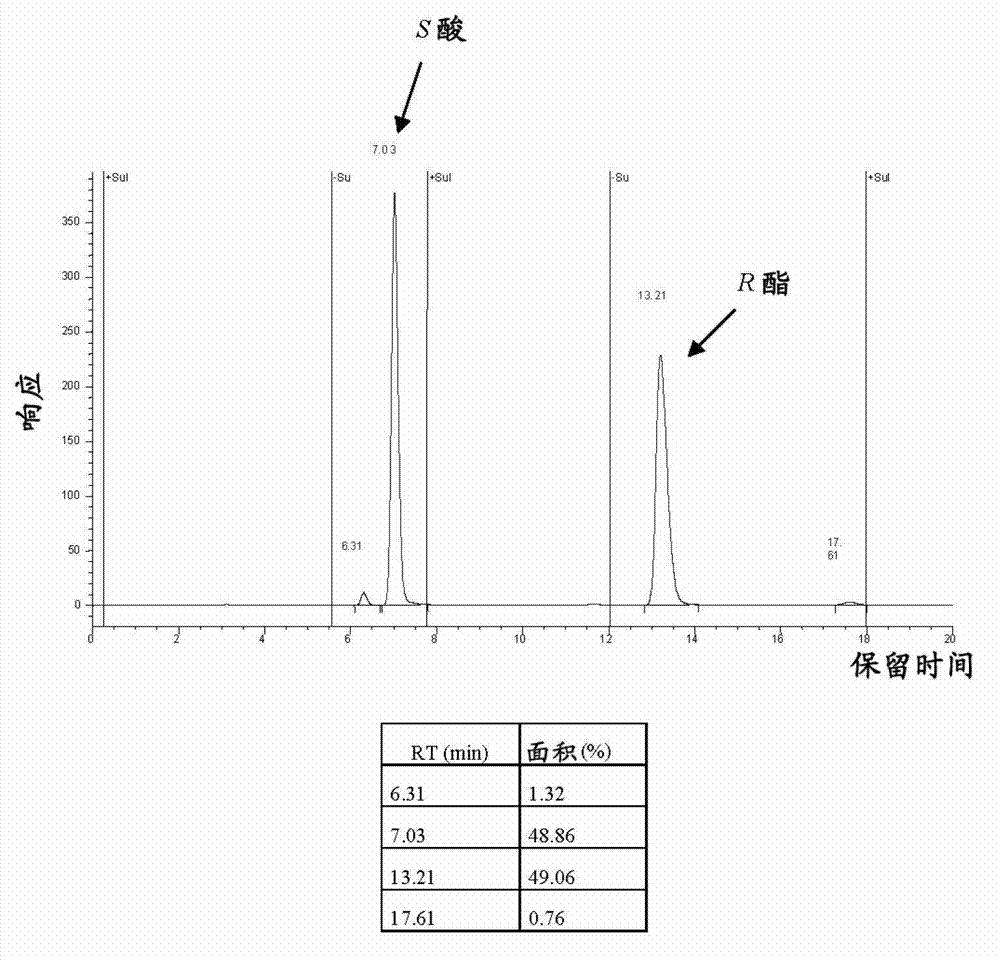
[0136] The chiral phase HPLC chromatograms of the racemic compound and the product after 48 hours are shown in figure 1 and 2 shown.
[0137] After 48 hours, optically pure ester and acid were seen to be provided at an optimal acid...
Embodiment 3
[0141] Example 3 : Methyl 3,4-dimethoxybicyclo[4.2.0]oct-1,3,5-triene-7-carboxylate
[0142] Suspend methyl (7R)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-triene-7-carboxylate (445 mg) (ee>96%) in isopropyl Alcohol (2.5 mL), and diazabicycloundecene (58 μl-1.5 eq) was added.
[0143] The reaction mixture was heated at 65°C for 2 hours. Complete racemization was observed at the end of 2 hours of the ester reaction.
[0144] Analysis conditions:
[0145] IC250*4.6 column
[0146] 30% anhydrous ethanol + 0.1% TFA + 70% heptane + 0.1% TFA
[0147] 1ml / min, 25°C288nm
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com