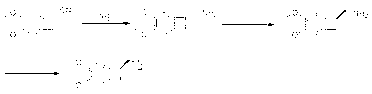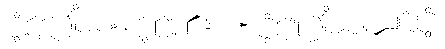Novel synthetic method of ivabradine and novel intermediate product of ivabradine
A new and synthetic method technology for ivabradine, which is applied in the field of synthetic methods and its new intermediate products, and can solve the problems of large solvent consumption, high preparation cost and difficult preparation of ivabradine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a 500mL reaction flask, add 30.0g N-methyl-3-chloropropylamine hydrochloride, 200mL dichloromethane, 30mL water, stir for 10min, then add 33.0g sodium carbonate, stir for 30min, filter, and wash with dichloromethane cake, and the filtrate was dried with anhydrous sodium sulfate for later use.
[0035] Add 400mL of dichloromethane to a 1L reaction flask, add 41.6g of (1S)-4,5-dimethoxybenzocyclobutane-1-carboxylic acid, 42.5g of N,N'-dicyclohexyl carbon under stirring Imide (DCC), stir for 10 minutes after addition, cool down in an ice-water bath to below 10°C, add the dichloromethane solution of N-methyl-3-chloropropylamine dropwise, control the feeding rate so that the temperature of the reaction solution does not exceed 10°C After the addition, continue to react at 10°C~15°C, a large amount of white solids are produced, the raw materials are completely reacted in about 2 hours, the reaction solution is filtered, the filter cake is washed with an appropriate amount ...
Embodiment 2
[0037] In a 500 mL reaction flask, add 30.0 g of N-methyl-3-chloropropylamine hydrochloride, 200 mL of dichloromethane, 30 mL of water, stir for 10 min, then add 33.0 g of sodium carbonate, stir for 30 min, filter, The filter cake was washed with dichloromethane, and the filtrate was dried over anhydrous sodium sulfate for later use.
[0038] Add 400 mL of dichloromethane into a 1 L reaction flask, add 41.6 g of (1S)-4,5-dimethoxybenzocyclobutane-1-carboxylic acid while stirring, and cool down to 0°C~5°C in an ice-water bath , began to add 28.5 g of thionyl chloride dropwise, controlled the rate of addition so that the temperature of the reaction solution did not exceed 10°C, removed the ice-water bath after dropping, stirred and reacted at room temperature for 2 h, evaporated the solvent to dryness under reduced pressure to obtain a brown oil, added 250 mL Dichloromethane dissolves, and the solution obtained after dissolving is added dropwise to the mixed solution of the dich...
Embodiment 3
[0040] In a 500 mL reaction flask, under inert gas protection, add 200 mL of anhydrous tetrahydrofuran, 9.8 g of (1S)-[N-(3-chloropropyl)-N-methyl]carbamoyl prepared in Example 1 or 2 -4,5-dimethoxybenzocyclobutane, stir to dissolve, add 6.3 g lithium tetrahydrogen aluminum to the reaction liquid in batches at room temperature, after the addition is complete, stir at room temperature for 1 h, then heat up to reflux for reaction 1 h, the raw material reaction of the sampling point plate is complete, the reaction solution is cooled to room temperature, and 10 mL of water is slowly added dropwise to the reaction solution to quench the reaction, then the reaction solution is transferred to a 2 L beaker, 500 mL of ethyl acetate is added, and then Anhydrous sodium sulfate was stirred and dried for 2 h, the desiccant was filtered off and the solvent was evaporated to dryness under reduced pressure to obtain 9.0 g of white solid, namely compound III: (1S)-[N-(3-chloropropyl)-N-methyl] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com