Medicinal composition containing silver ester medicine and ibobulodine
A composition and technology of isosorbide dinitrate, applied in directions such as pharmaceutical combinations, active ingredients of nitro compounds, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] Protective effects of isosorbide mononitrate and ivabradine on chronic myocardial ischemia in rats induced by large doses of isoproterenol Experimental purpose:
[0009] To observe the protective effects of isosorbide mononitrate, ivabradine and their combination on chronic myocardial ischemia induced by high dose isoproterenol in rats.
[0010] experimental method:
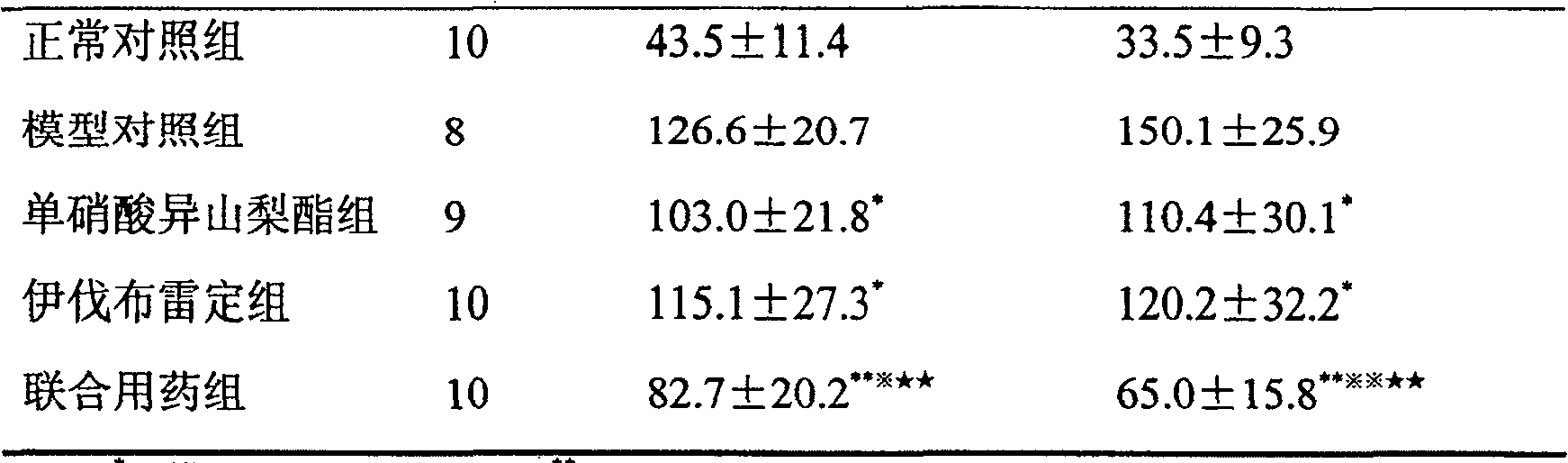
[0011] 50 male SD rats were randomly divided into 5 groups, 10 in each group:
[0012] 1. Normal control group: 50 μL / (kg·d) normal saline was injected subcutaneously for 7 days, and 10 mL / (kg·d) normal saline was administered by intragastric administration for 21 days at the same time.
[0013] 2. Model group: subcutaneous injection of isoproterenol 5 mg / (kg·d) to make a chronic myocardial ischemia model for 7 consecutive days; at the same time, intragastric administration of 10 mL / (kg·d) normal saline for 21 consecutive days.
[0014] 3. Isosorbide mononitrate group: Modeling was the same as before, an...
Embodiment 2
[0029] Protective effects of isosorbide mononitrate and ivabradine on chronic myocardial ischemia in minipigs
[0030] Purpose:
[0031] To observe the protective effects of isosorbide mononitrate, ivabradine and their combination on chronic myocardial ischemia in minipigs.
[0032] experimental method:
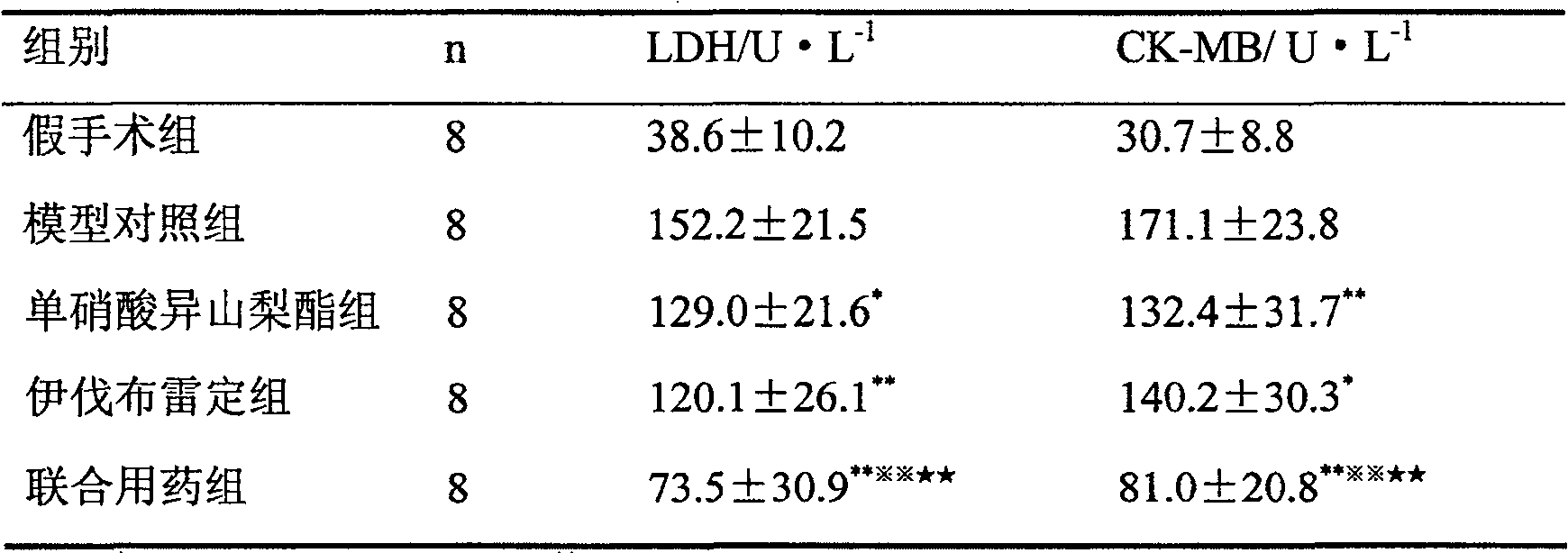
[0033] About 40 Chinese experimental mini-pigs were taken, and the Ameroid chronic narrowing ring was placed on the proximal end of the left anterior descending coronary artery through thoracotomy. Coronary angiography was performed 5 weeks later, and the anterior descending artery showed obvious stenosis, and the degree of stenosis was selected as 85. From % to 100% of miniature pigs, blood was taken from the large ear vein, and the activities of lactate dehydrogenase (LDH) and creatine kinase MB subtype (CK-MB) in the blood were detected according to the method described in the kit instructions. According to LDH and CK The sum of plasma levels of -MB Miniature pigs were r...
Embodiment 3
[0049] Ivabradine Isosorbide Mononitrate Tablets
[0050] Isosorbide Mononitrate 15g
[0051] Ivabradine 5g
[0052] Starch 50g
[0053] L--HPC 20g
[0054] 10% starch slurry appropriate amount
[0056] Preparation Process:
[0057]Pass the isosorbide mononitrate, ivabradine, starch and L-HPC in the prescription through a 100-mesh sieve, mix well, add 10% starch slurry to granulate in an appropriate amount, dry below 50°C, and granulate with a 18-mesh sieve. Add the prescribed amount of magnesium stearate, mix well, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com