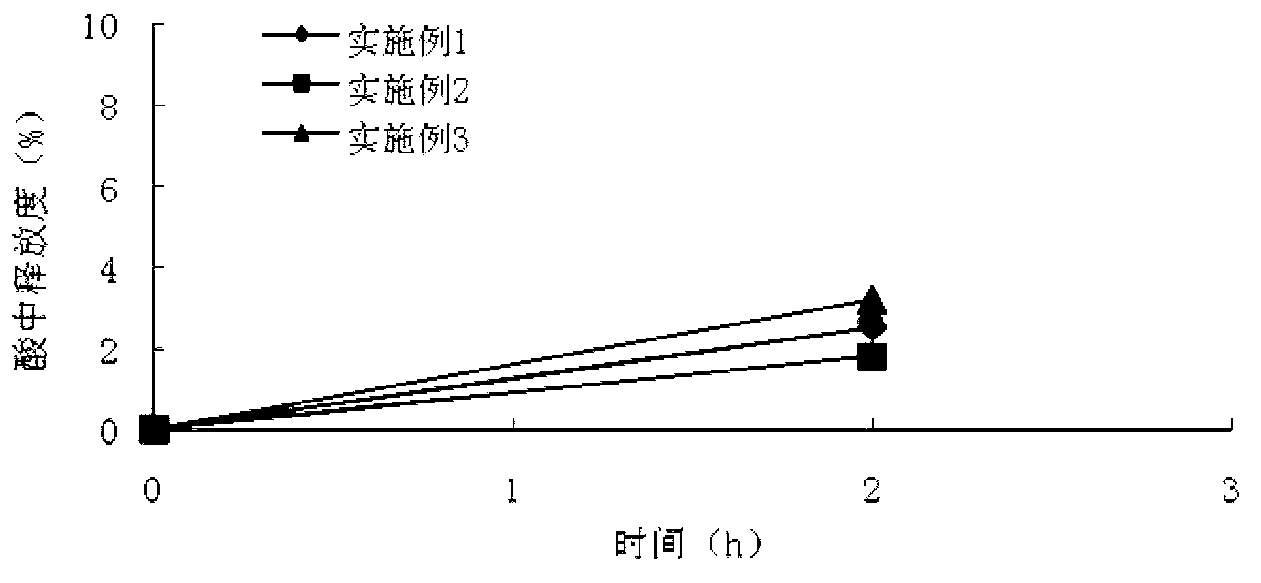
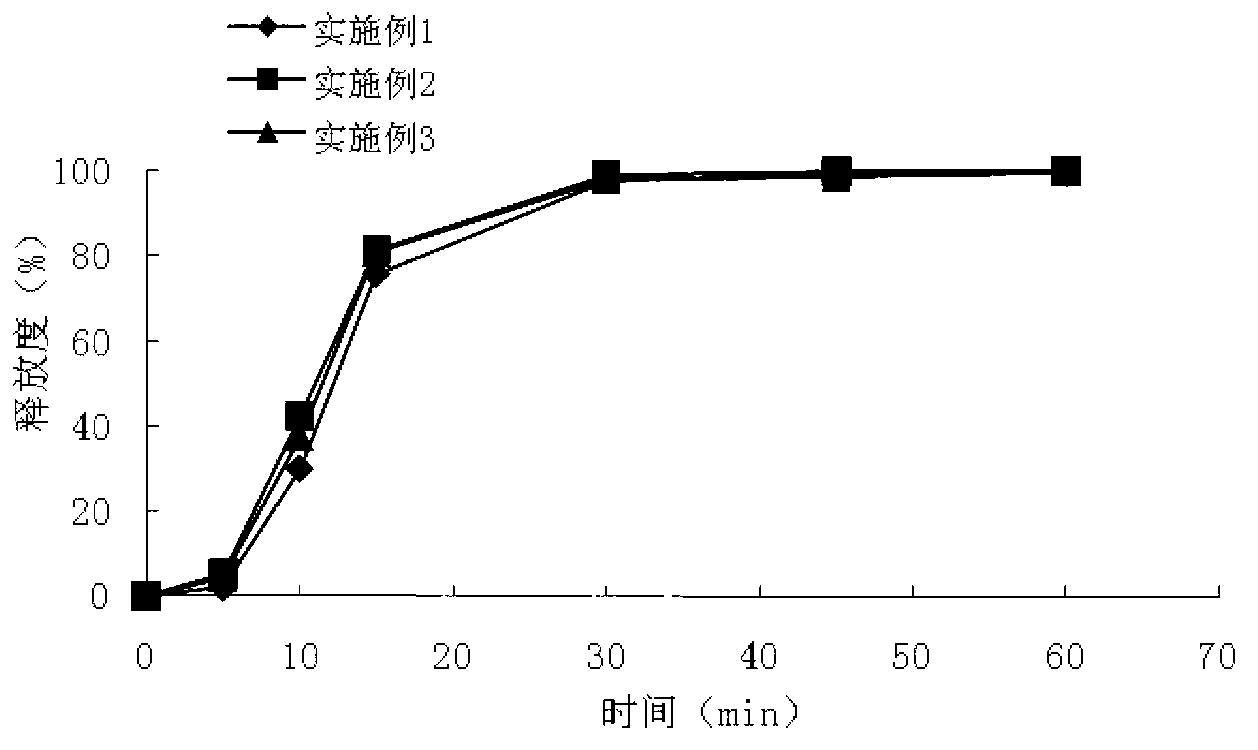
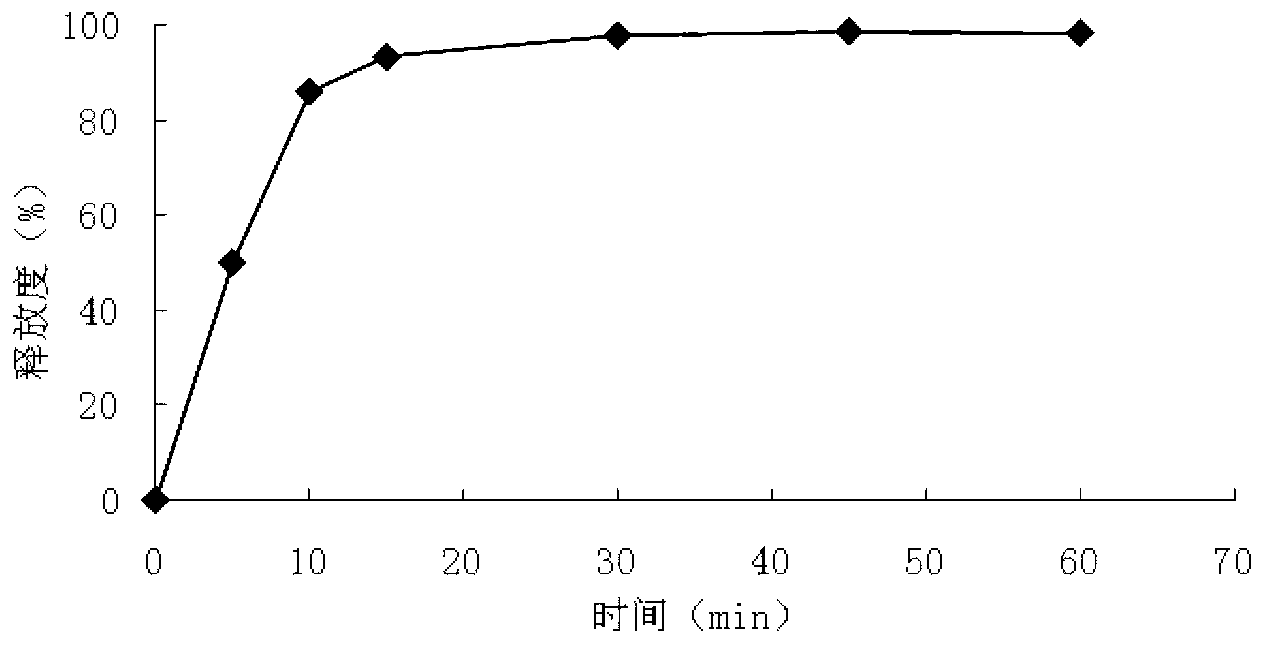
Compound isosorbide mononitrate aspirin sustained-release capsule preparation and preparation method
A technology of isosorbide dinitrate and aspirin, which is applied to pharmaceutical formulations, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., can solve the problems of poor drug release reproducibility, slow initial drug release, and large individual differences.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0057] Embodiment 2, compound recipe isosorbide mononitrate aspirin sustained-release capsule preparation, produces 1000 capsules
[0058] Ball core prescription:
[0059]
[0060] ER gown prescription:
[0061] Ethylcellulose N100 30g
[0062] Povidone K30 5g
[0063] Ethanol 1000ml
[0064] Film Coating Prescription:
[0065] Opadry Gastric Coating Powder 12g
[0066] water 88g
[0067] Prescription of aspirin enteric-coated granules:
[0068] Aspirin 75g
[0069] Enteric coating prescription:
[0070]
Embodiment 3
[0071] Embodiment 3, compound recipe isosorbide mononitrate aspirin sustained-release capsule preparation, produces 1000 capsules
[0072] Ball core prescription:
[0073]
[0074] ER gown prescription:
[0075] Ethylcellulose N100 30g
[0076] Povidone K30 6g
[0077] Ethanol 1000ml
[0078] Film Coating Prescription:
[0079] Opadry Gastric Coating Powder 12g
[0080] water 88g
[0081] Prescription of aspirin enteric-coated granules:
[0082] Aspirin 75g
[0083] Enteric coating prescription:
[0084]
[0085] The compound recipe isosorbide mononitrate capsule preparation described in above embodiment 1, embodiment 2, embodiment 3 is made by following method:
[0086] Step 1, preparation of isosorbide mononitrate slow-release preparation:
[0087] 1.1, the preparation of 1% sodium carboxymethyl cellulose 10% ethanol solution: take the sodium carboxymethyl cellulose of prescription quantity and disperse evenly in 10% ethanol of prescription quantity, then add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com