Special target medicine and its use
A targeted and specific technology, applied in the direction of drug combination, application, antineoplastic drugs, etc., can solve the problems of not being able to effectively reduce the adverse reactions of IFN, and not being able to improve the curative effect of IFN at the dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 Design of specific targeting interferon amino acid sequence
[0052] 1. The X sequence selects the 1st to 50th amino acid sequence (SEQ ID NO: 3) of the large surface protein of the HBV strain FMC#97, which includes the widely confirmed specific binding to the surface of liver cells and mediates HBV infection of liver cells. key sequence.
[0053] 2. L selects a sequence composed of flexible amino acids (SEQ ID NO: 4) to reduce the interaction between X and Y in terms of protein conformation and function.
[0054] 3. The Y sequence selects the full-length amino acid sequence of IFNα-2b (SEQ ID NO: 5).
[0055] 4. X and Y are respectively located at the N-terminus and C-terminus of the L sequence, and the overall sequence formed is shown in SEQ ID NO:6.
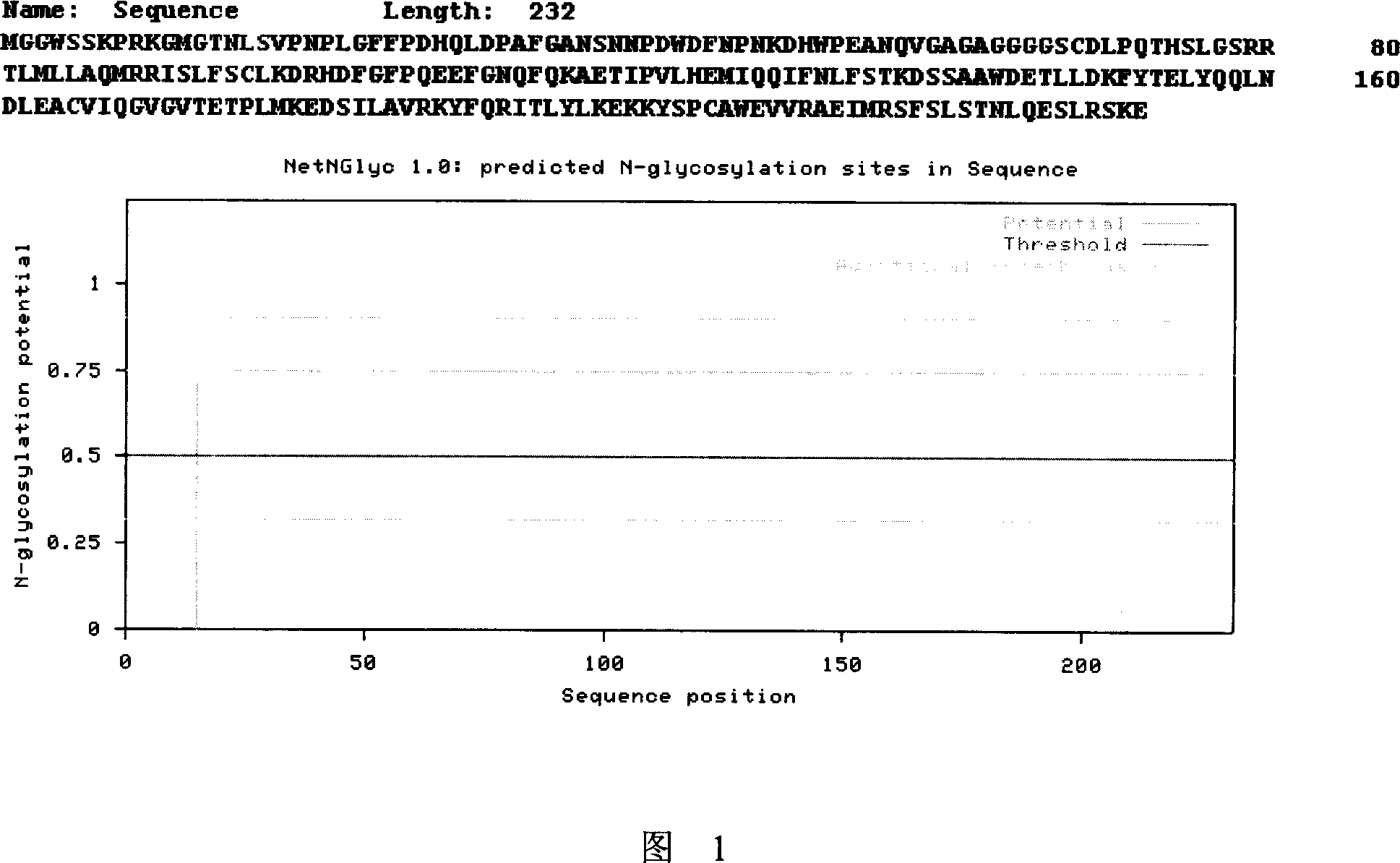
[0056] 5. Carry out glycosylation site analysis on SEQ ID NO: 6 (see Figure 1), wherein the 15th amino acid asparagine is a possible N-linked glycosylation modification site, which is subjected to similar...
Embodiment 2
[0057] The design of the DNA sequence of embodiment 2 coding specific targeting interferon
[0058] 1. Select Pichia pastoris as the expression system for specific targeting interferon. Pichia pastoris is the most successful eukaryotic expression system for exogenous protein expression (Cereghino J L, Cregg J M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris [J]. FEMS MicrobiolRev, 2000, 24 (1): 45 -66.). It not only has the characteristics of rapid propagation, low cost and convenient operation of the prokaryotic expression system, but also has the advantages of the eukaryotic expression system with correct processing and folding of exogenous proteins and moderate glycosylation. Therefore, it is more and more widely used for the mass expression of proteins. At present, more than 200 kinds of proteins have been expressed in this expression system (Cregg JM, Cereghino JL, Shi JY, et al. Recombinant protein expression in Pichia pastoris[J].Mol Biot...
Embodiment 3
[0061] Embodiment 3 Construction of specific targeting interferon yeast expression vector
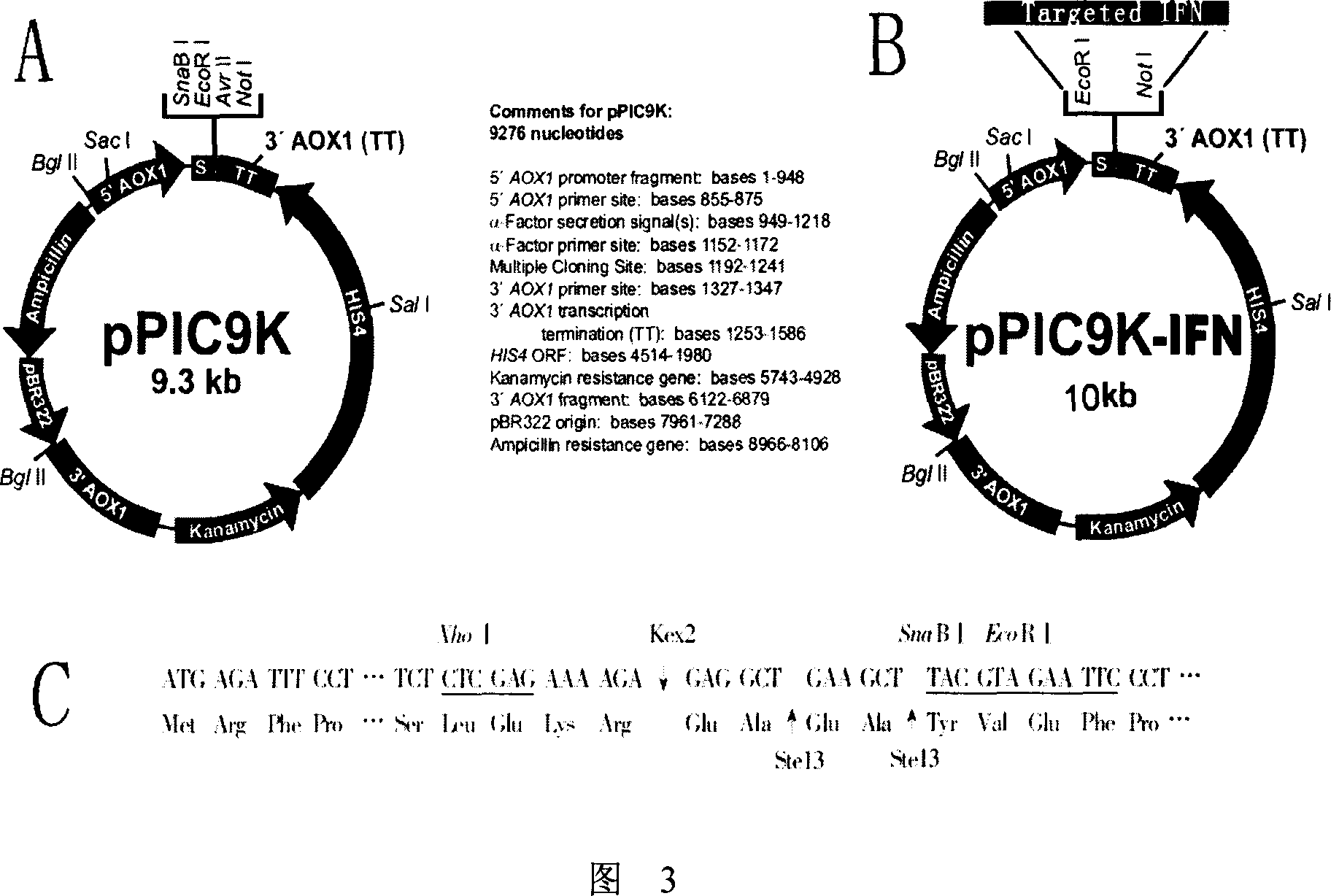
[0062] 1. Select pPIC9K as the yeast expression vector for specific targeting interferon. pPIC9K is a multi-copy secretory expression vector introduced by Invitrogen, with a size of 9.3kb (as shown in Figure 3A). The vector contains Escherichia coli replication origin Col E1 and two resistance genes of ampicillin and kanamycin, and the kanamycin resistance gene can also make Pichia pastoris resistant to G418, which can be used for screening of multiple copy insertions . The promoter of the alcohol oxidase gene (pAOX1) is used in the vector as an inducible promoter, which is inhibited by glucose and starts transcription and translation after being induced by methanol, which is very suitable for the expression of foreign genes. pPIC9K uses the secretion signal of a mating factor leader sequence to secrete the target gene product to the extracellular space. Since Pichia pastoris only se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com