Preparation method for Ivabradine
A technology of compound and addition salt, applied in the field of preparation of ivabradine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
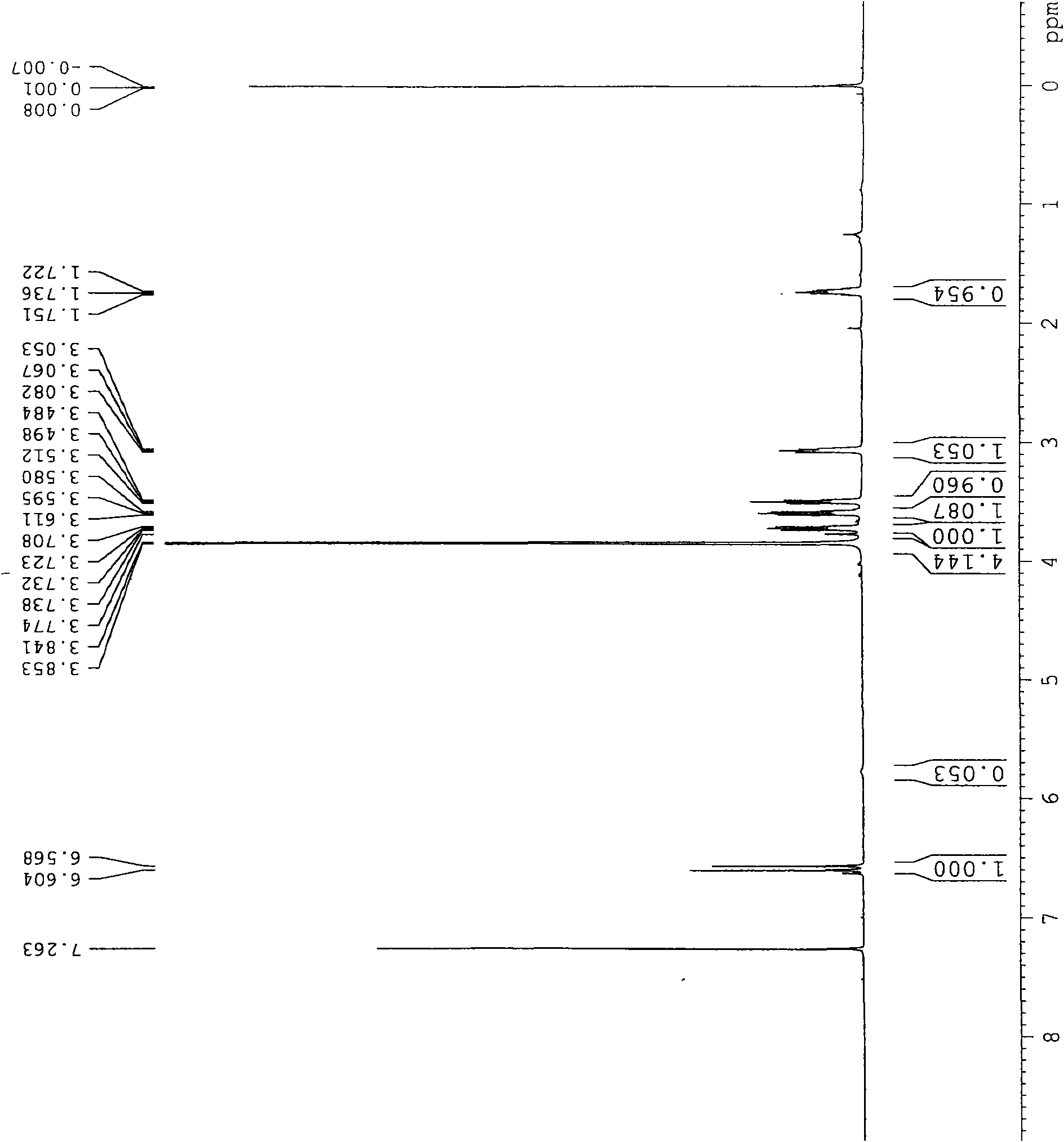
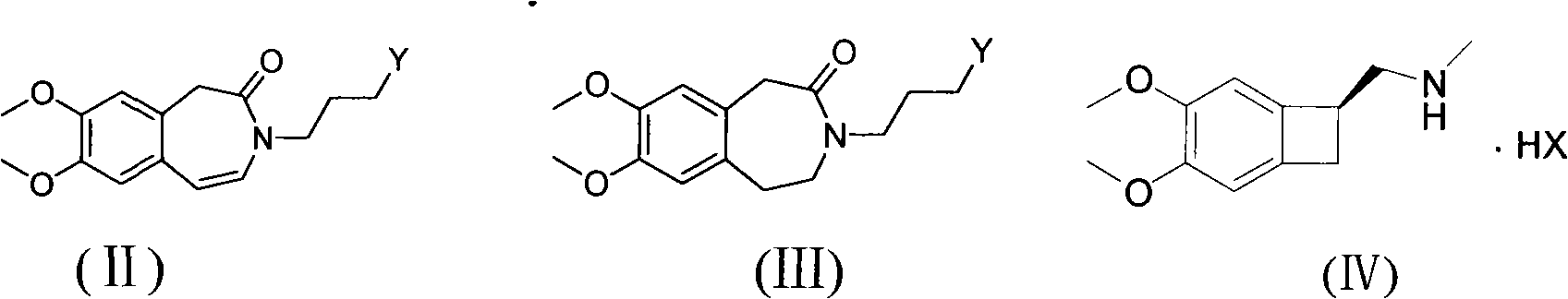
[0035] Example 1: 3-(3-hydroxypropyl)-7,8-dimethoxy-4,5-dihydro-1H-benzazepine-2(3H)-one 2.3g3-(3- Hydroxypropyl)-7,8-dimethoxy-1H-benzazepine-2(3H)-one and 1.8g 10% Pd / C were added to 20mL methanol, and then slowly introduced into the system h 2 , so that the bubbles in the system are uniform. Heat the system to react at 40°C for 24h. Pd / C was recovered by suction filtration, and the filtrate was rotary evaporated to obtain 1.97 g of white solid, yield: 85%, melting point 102.5°C. 1H-NMR (CDCl 3 , 400MHz), δ1.72-1.75(t, 2H), 3.05-3.08(t, 2H), 3.48-3.51(t, 2H), 3.58-3.61(t, 2H), 3.71-3.74(q, 2H) , 3.84-3.85 (d, 8H), 6.57-6.60 (d, 2H). The NMR picture is attached figure 1
Embodiment 2
[0036] Embodiment 2: the hydrochloride preparation of ivabradine
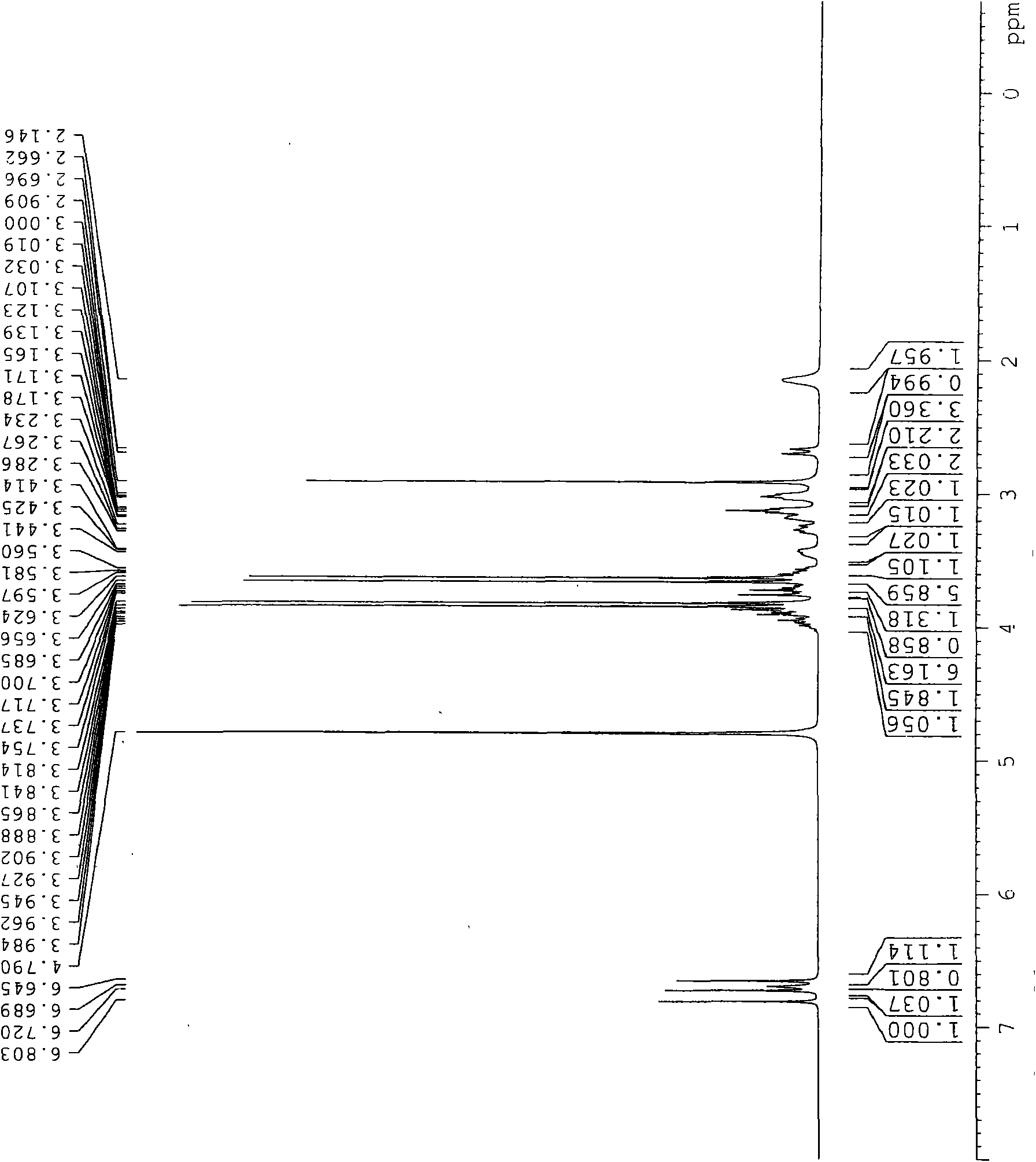
[0037] 0.4g 3-(3-hydroxypropyl)-7,8-dimethoxy-4,5-dihydro-1H-benzazepin-2(3H)-one and 0.3g (S)-( 4,5-dimethoxy-1,2-dihydrobenzocyclobutyl-1-yl)-N-methylmethanamine was added to 10 mL of acetone, and then 0.2 g KI and 0.5 g K 2 CO 3 , started stirring and heated to reflux. The reaction was stopped after 24 h of reaction. Suction filtration, the filtrate was spin-dried to obtain a viscous solid, the solid was dispersed in water, and a filter cake was obtained by suction filtration, and the filtrate was discarded. It was then dissolved in ethyl acetate, washed with 3N HCl solution, adjusted to pH 7-8 with sodium hydroxide solution, dried over anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to obtain an oily substance. The oil was dispersed in ethyl acetate to form monohydrochloride, and recrystallized from methanol and acetone to obtain 0.5 g of white crystalline powder with a yield o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com