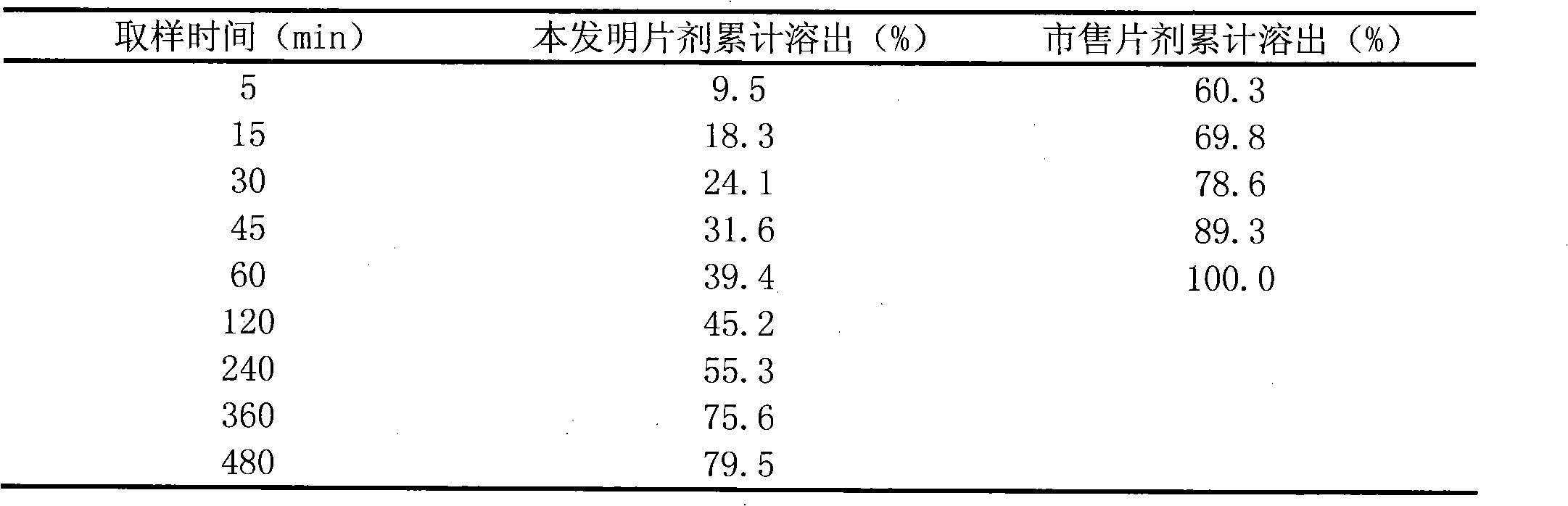
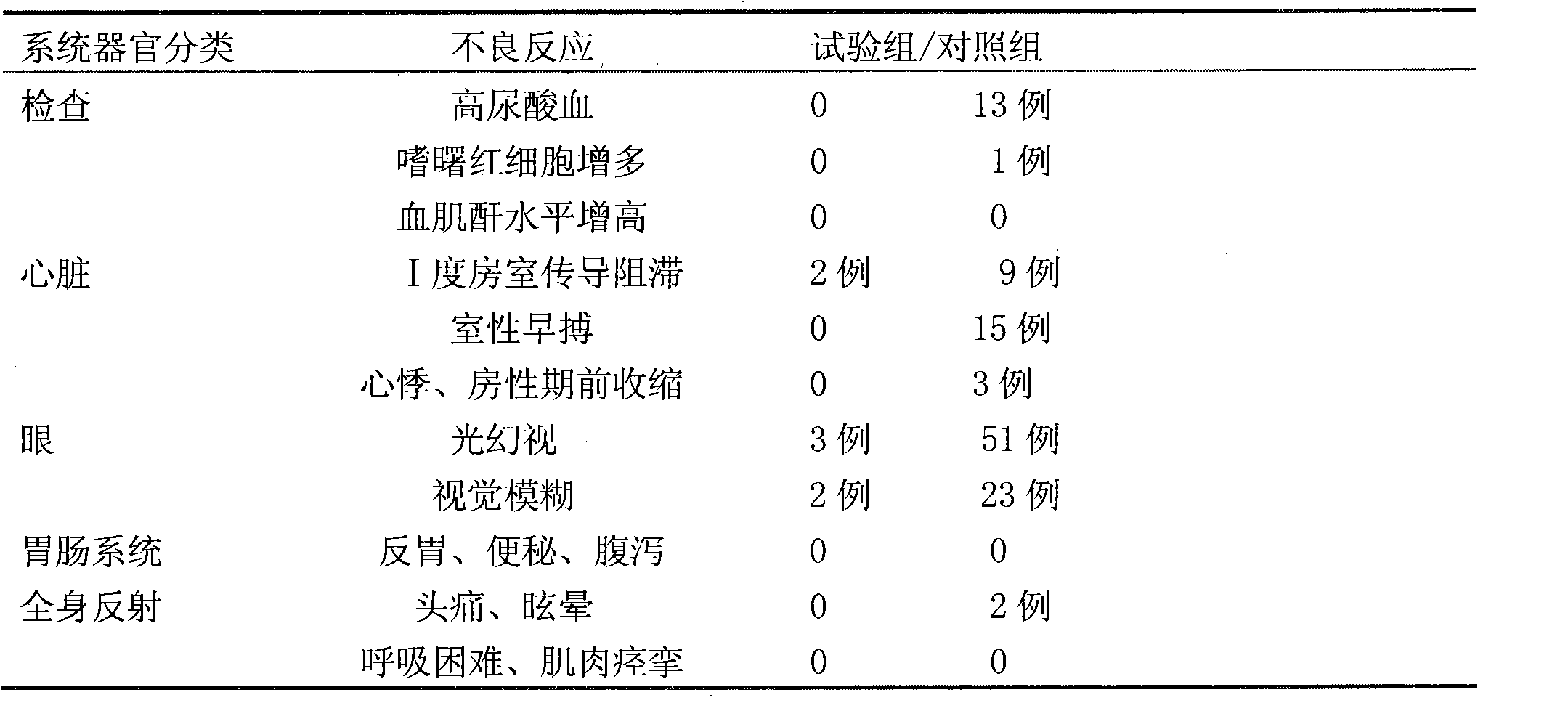
Ivabradine preparation or ivabradine medicinal salt solid preparation and preparation method thereof
A technology of solid preparations and medicinal salts, applied in the field of medicine, can solve problems such as unfavorable left ventricular systolic function, achieve the effects of avoiding sudden release of drugs, overcoming dose dependence, and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 ivabradine hydrochloride solid preparation
[0022] (1) After mixing 15g of xanthan gum and 10g of silk fibroin, stir and melt at 55°C and keep warm, slowly add 10g of micropowdered silica gel while stirring, so that the micropowdered silica gel is evenly dispersed, and the composition is obtained for use;
[0023] (2) make microemulsion with propoxylated methyl glucoside 15g, isobutanol 5ml, ethyl acetate 40ml, add ivabradine hydrochloride 175g (in terms of ivabradine) in the emulsion, place in 40 ℃ in water bath;
[0024] (3) Add the composition obtained in (1) into the microemulsion, stir until uniform, use this solution as the reaction solution, adjust the pH value of the reaction solution to 8.0 with 10% NaOH solution, add an appropriate amount of methanol, and react at constant temperature for 1 hour ;
[0025] (4) Cool the reaction solution to 0°C, add glutaraldehyde 0.08 times the volume of the reaction solution and continue to ...
Embodiment 2
[0027] The preparation of the solid preparation of embodiment 2 ivabradine or its pharmaceutically acceptable salt
[0028] (1) Mix 45g of xanthan gum and 10g of silk fibroin, stir and melt at 60°C and keep warm, slowly add 10g of micropowdered silica gel while stirring, so that the micropowdered silica gel is evenly dispersed, and the composition is obtained for later use;
[0029] (2) make microemulsion with propoxylated methyl glucoside 120g, isobutanol 40ml, ethyl acetate 320ml, add ivabradine hydrochloride 975g (in terms of ivabradine) in the emulsion, place in 60 ℃ in water bath;
[0030] (3) Add the composition obtained in (1) to the microemulsion, stir until uniform, use this solution as the reaction solution, adjust the pH value of the reaction solution to 10.0 with 10% NaOH solution, add an appropriate amount of methanol, and react at a constant temperature for 2 Hour;
[0031] (4) Cool the reaction solution to 0°C, add glutaraldehyde 0.125 times the volume of the ...
Embodiment 3
[0033] The preparation of the solid preparation of embodiment 3 ivabradine or its pharmaceutically acceptable salt
[0034] (1) After mixing 25g of xanthan gum and 10g of silk fibroin, stir and melt at 58°C and keep warm, slowly add 10g of micropowdered silica gel while stirring, so that the micropowdered silica gel is evenly dispersed, and the composition is obtained for use;
[0035] (2) Microemulsion was prepared with 60 g of propoxylated methyl glucoside, 20 ml of isobutanol, and 160 ml of ethyl acetate. Add 540 g of ivabradine hydrochloride (calculated as ivabradine) into the emulsion and place at 50° C. in a water bath;
[0036] (3) Add the composition obtained in (1) into the microemulsion, stir until uniform, use this solution as the reaction solution, adjust the pH value of the reaction solution to 9.0 with 10% NaOH solution, add an appropriate amount of methanol, and react at constant temperature for 1.5 hours ;
[0037] (4) Cool the reaction solution to 0°C, add g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com