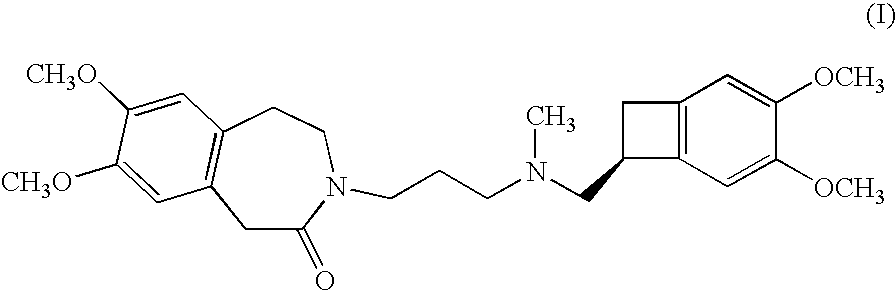
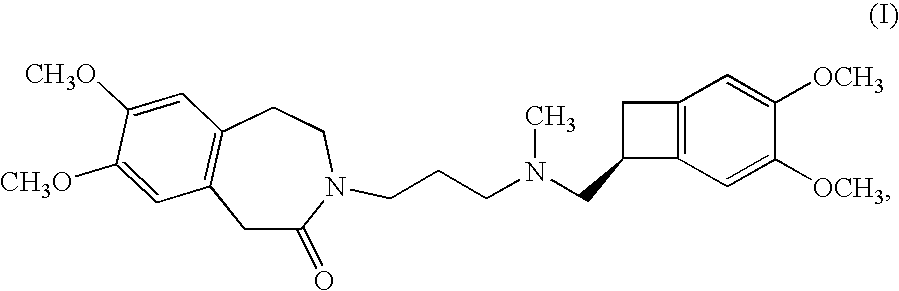
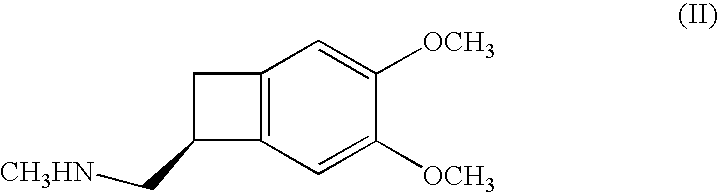
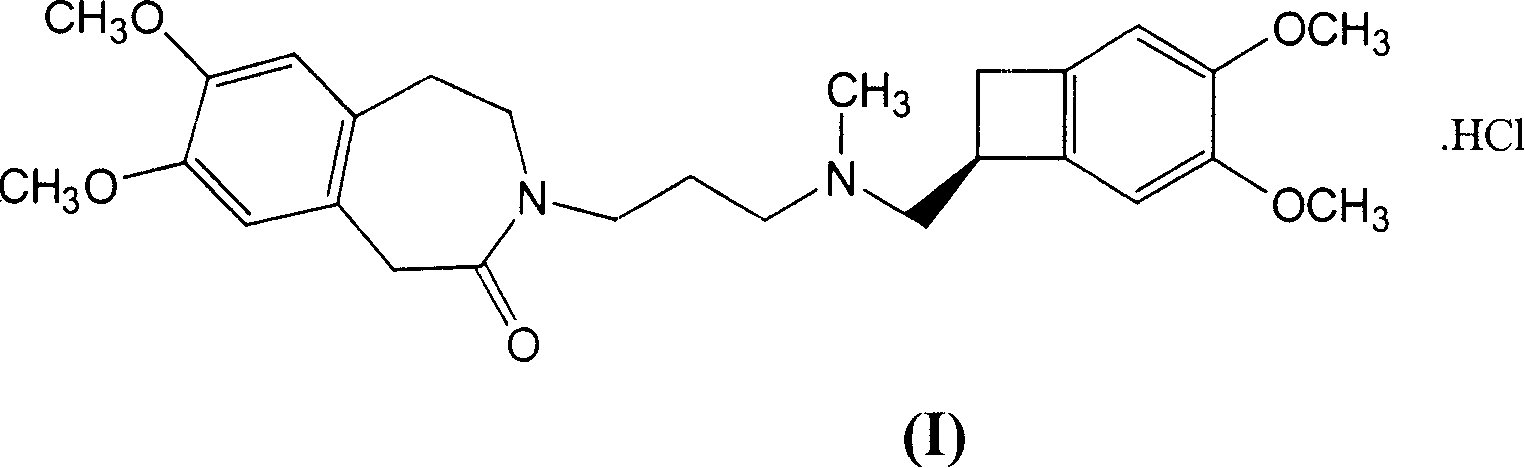
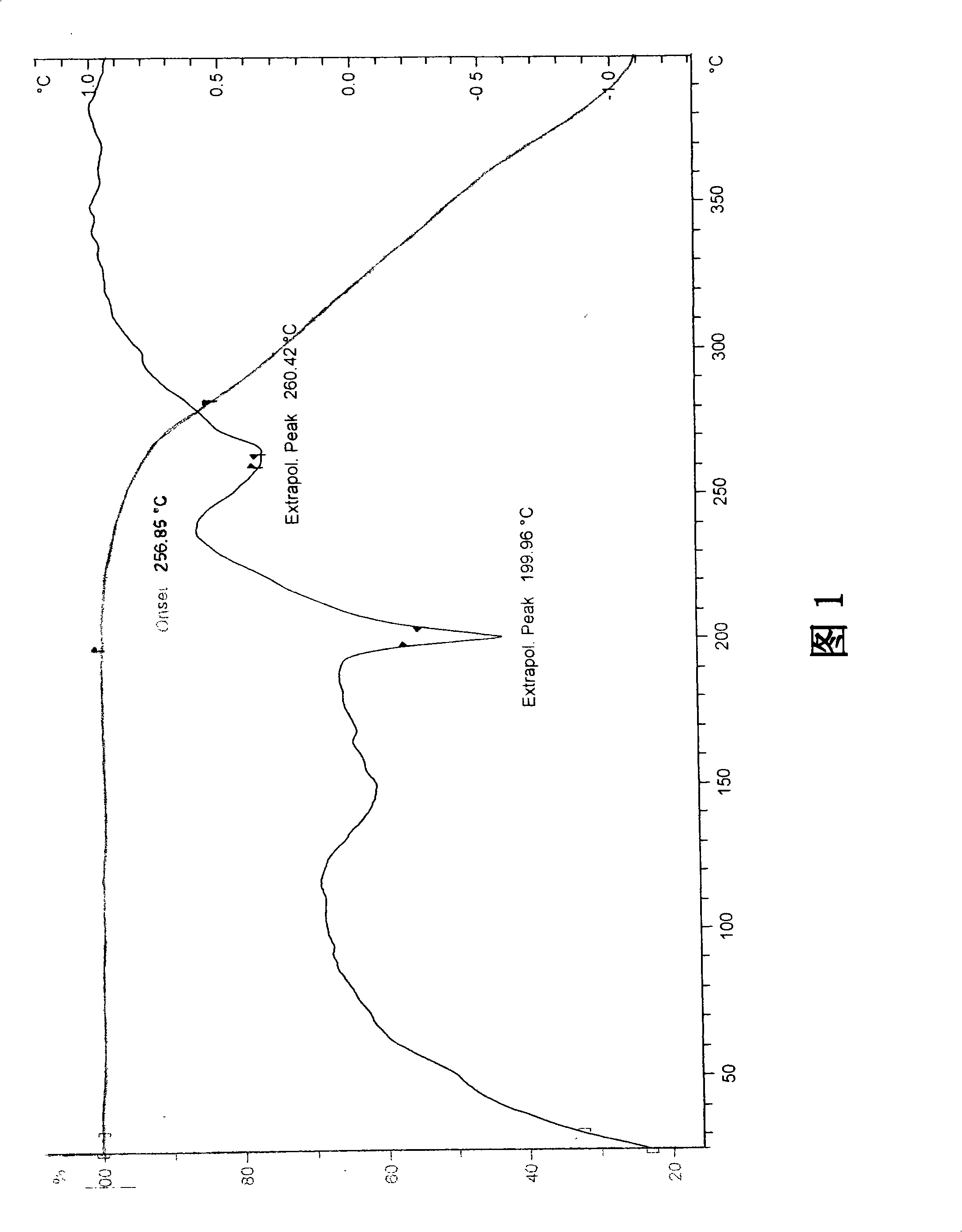
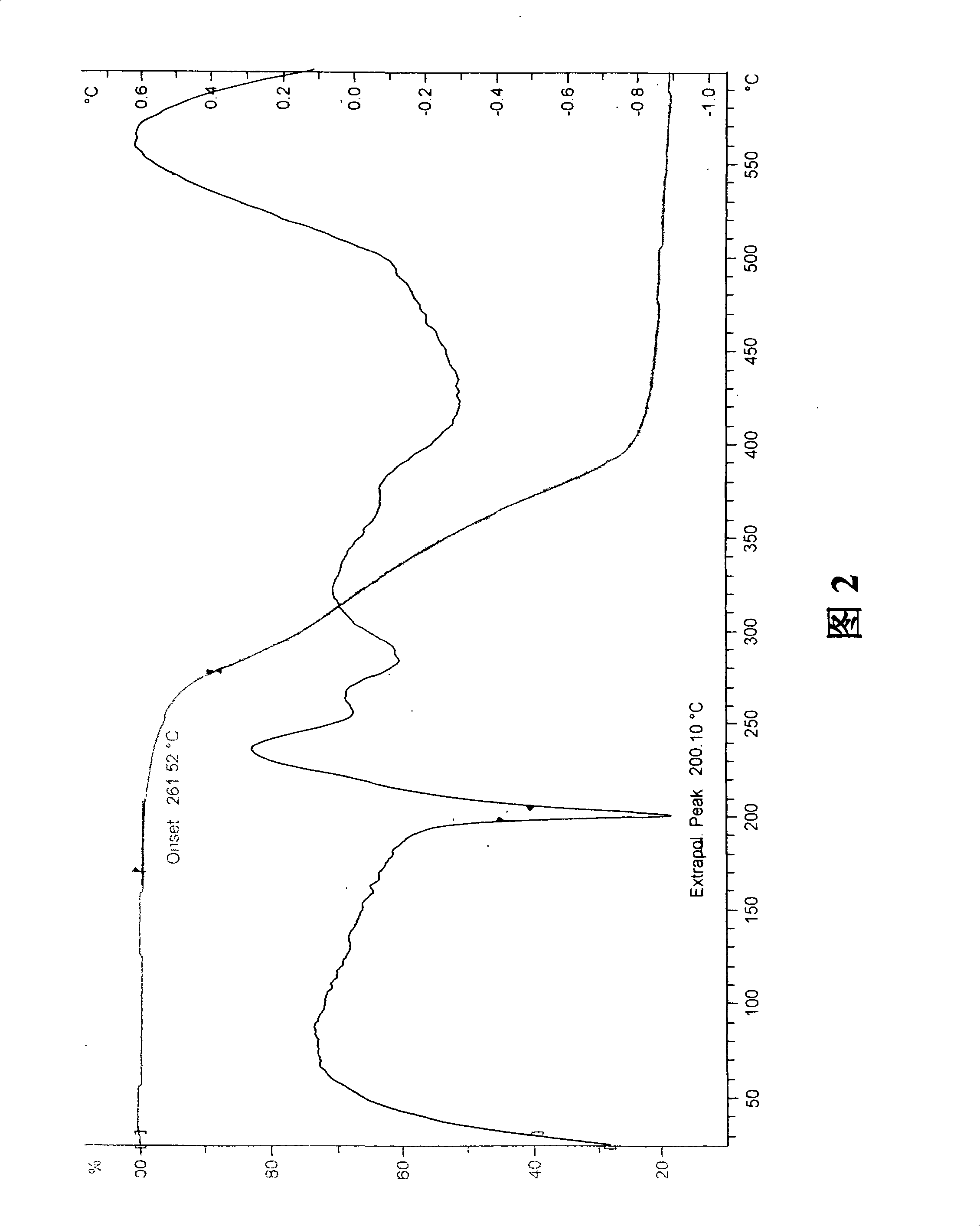
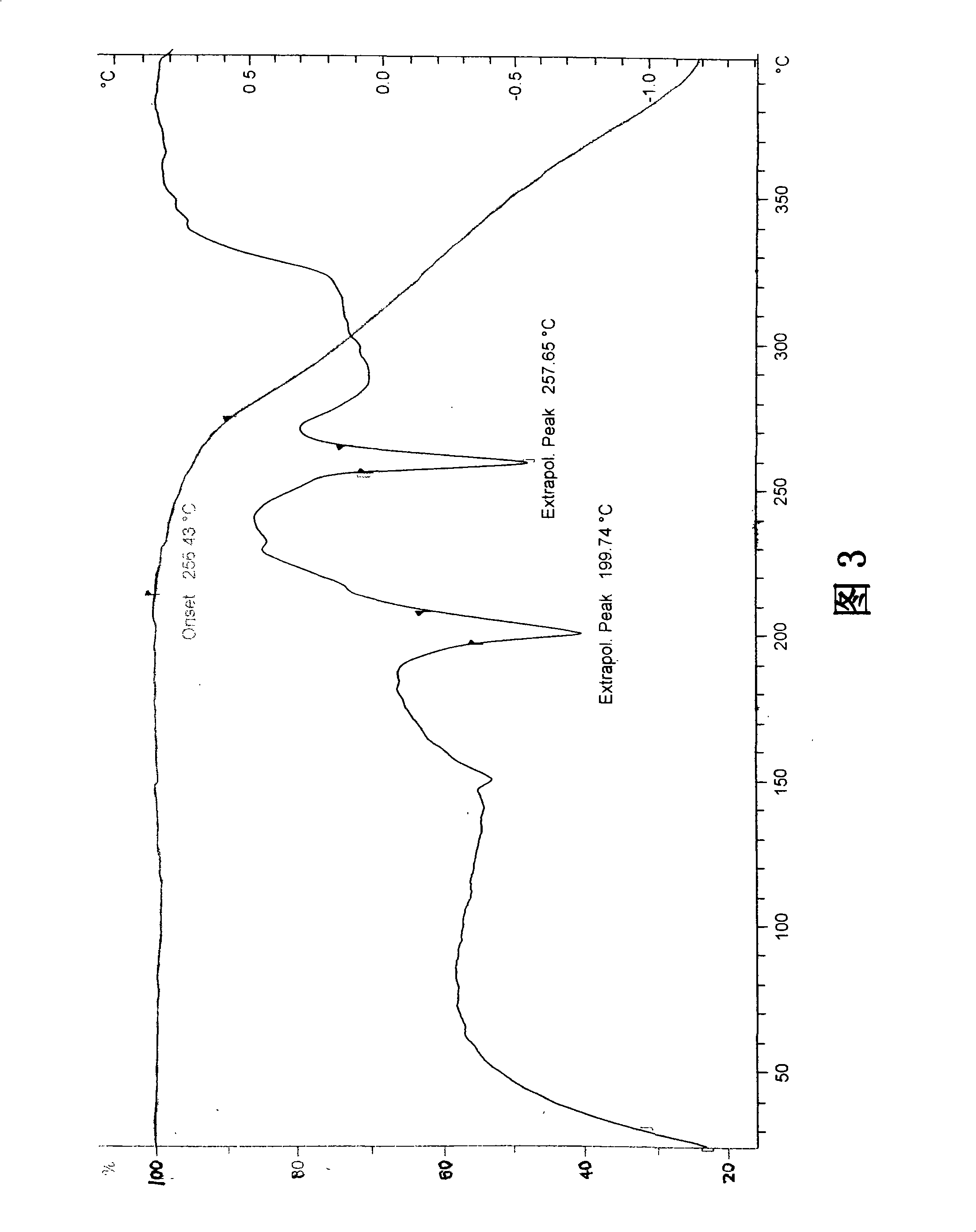
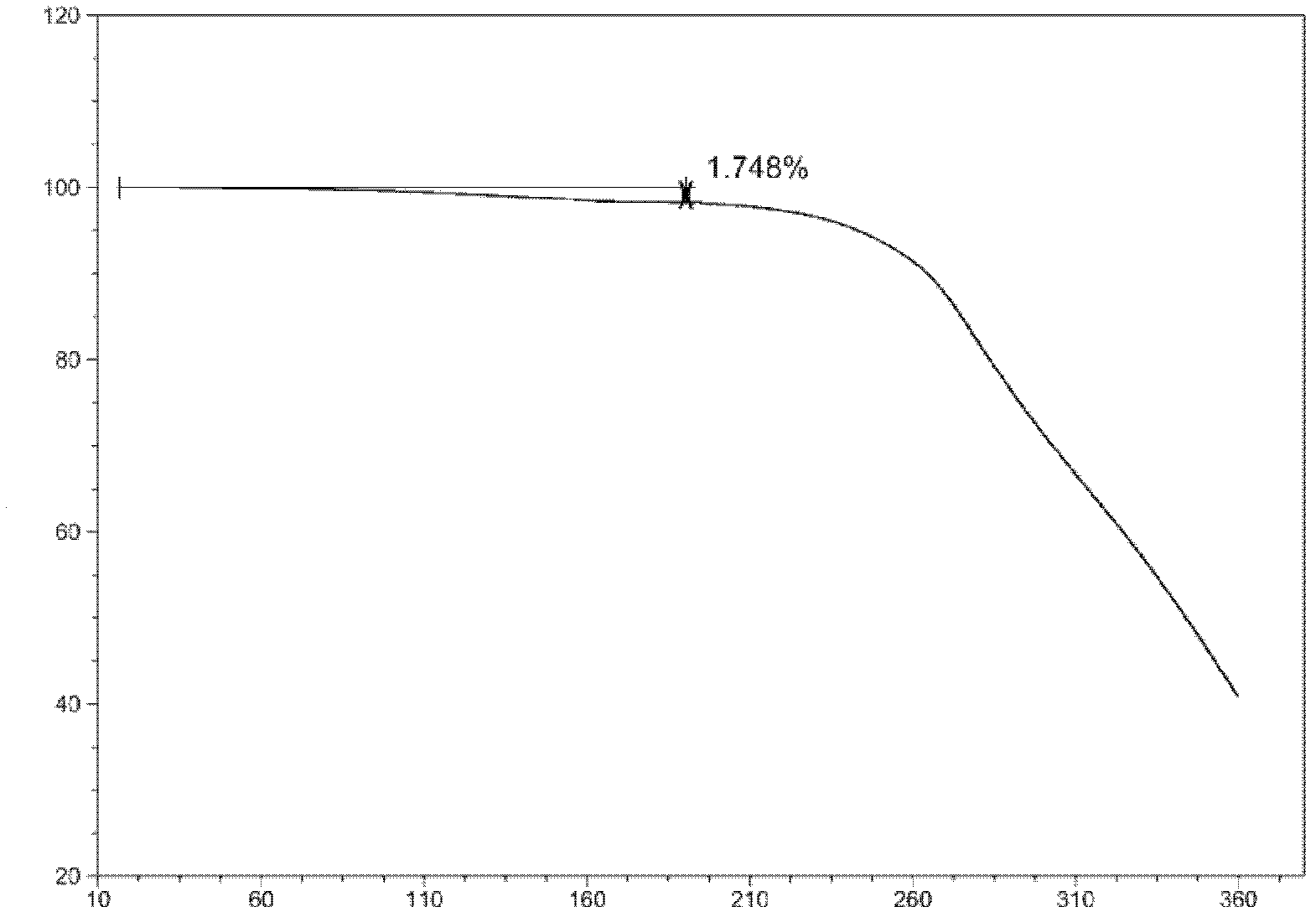
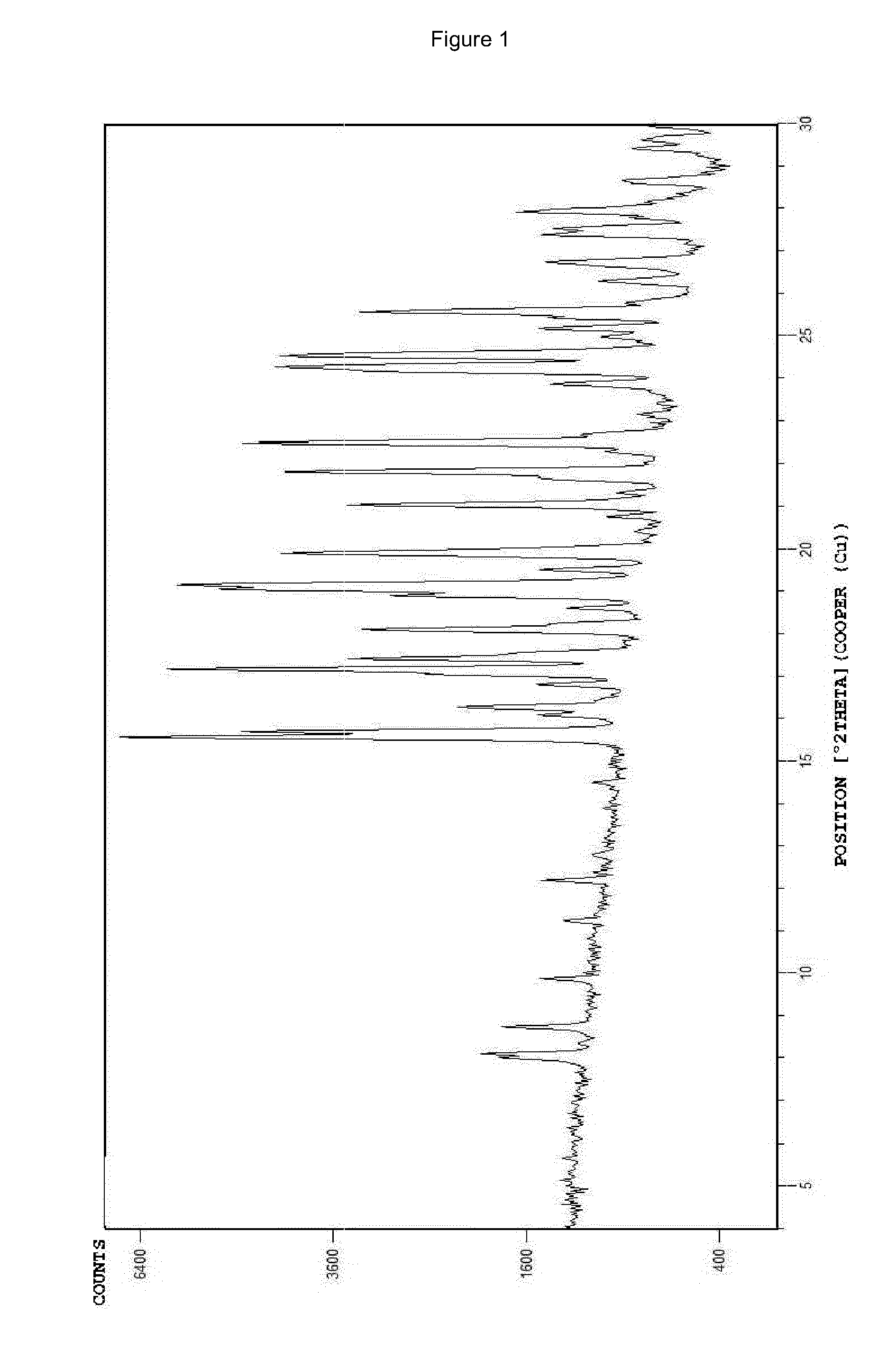
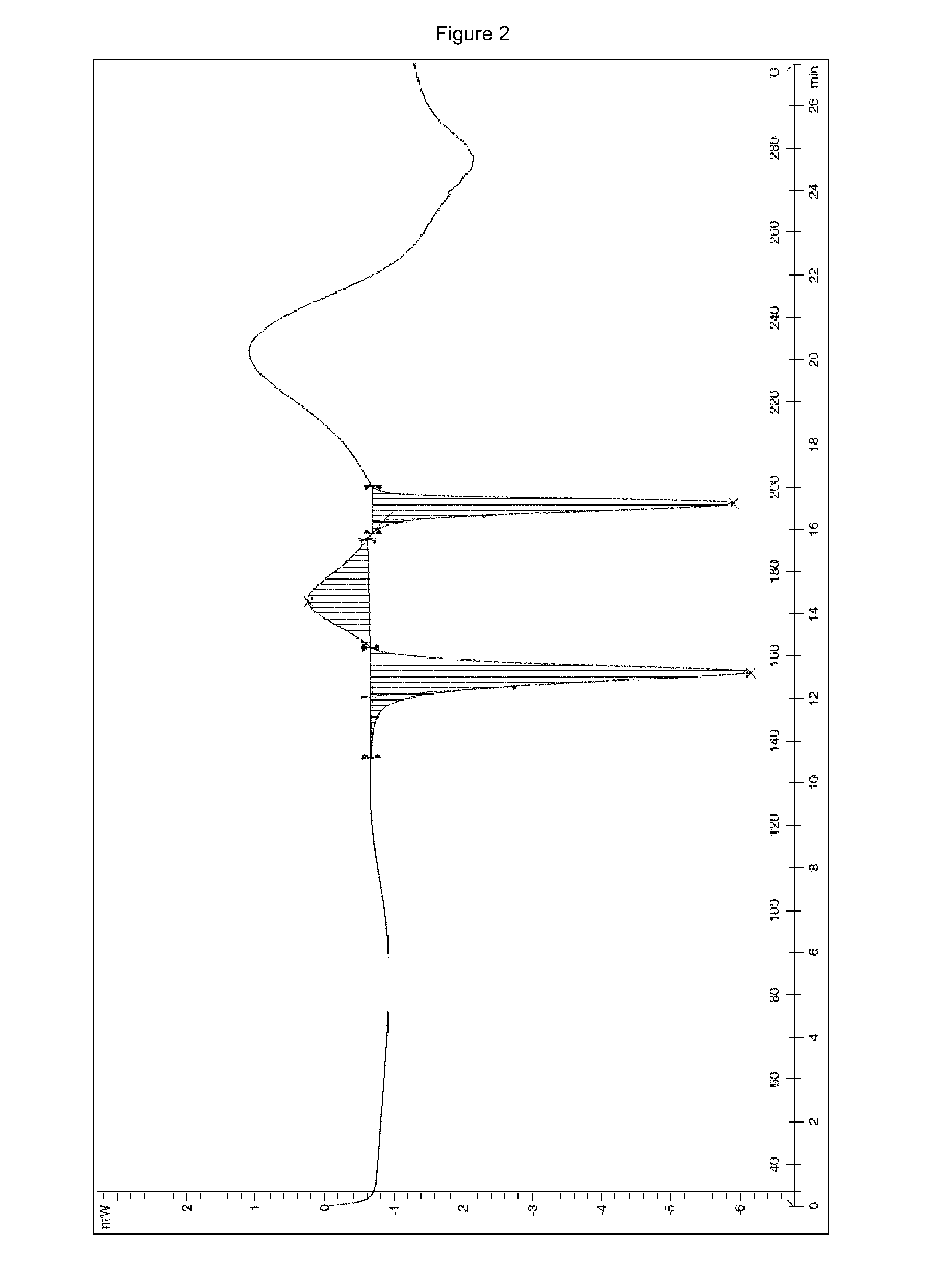
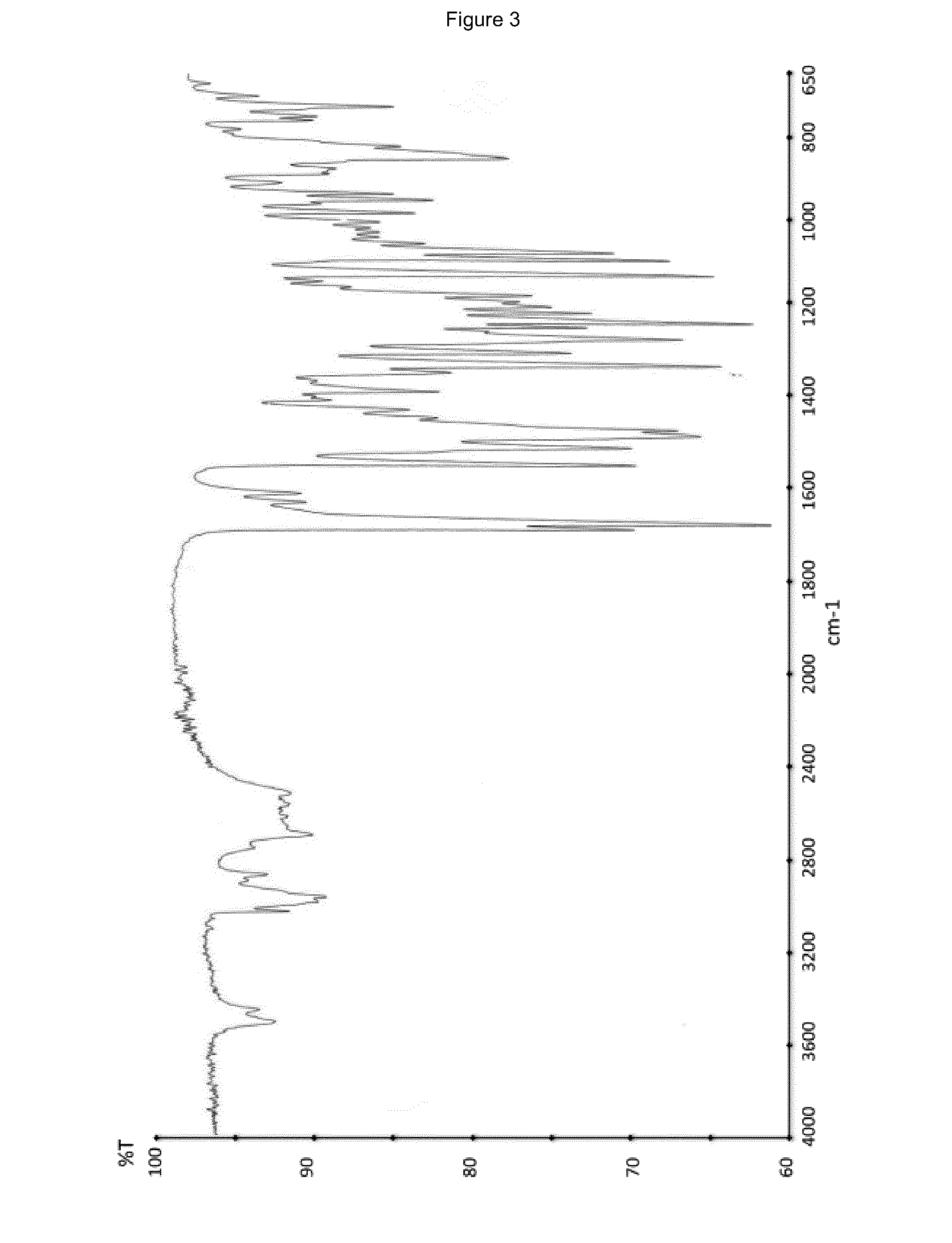
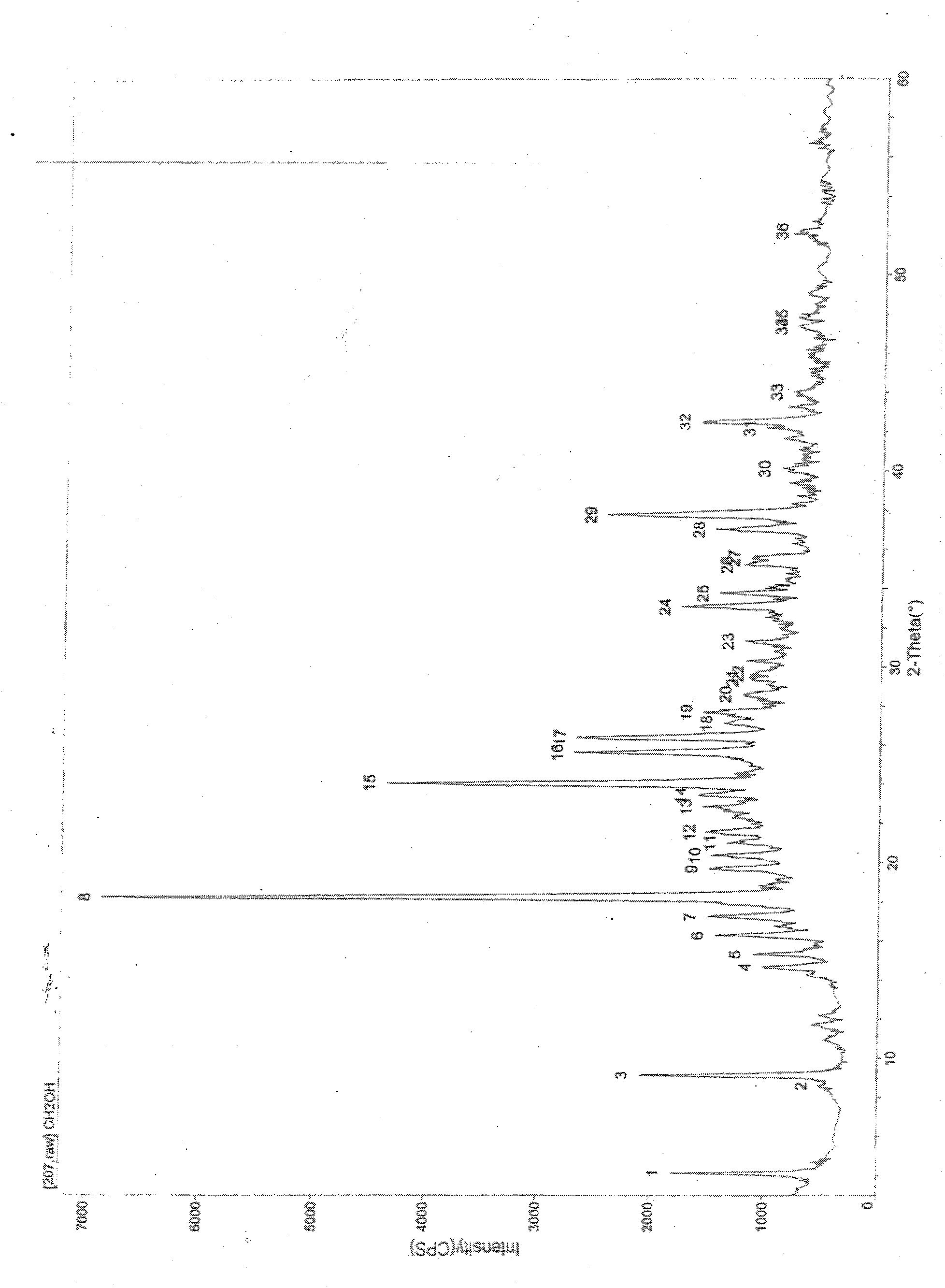
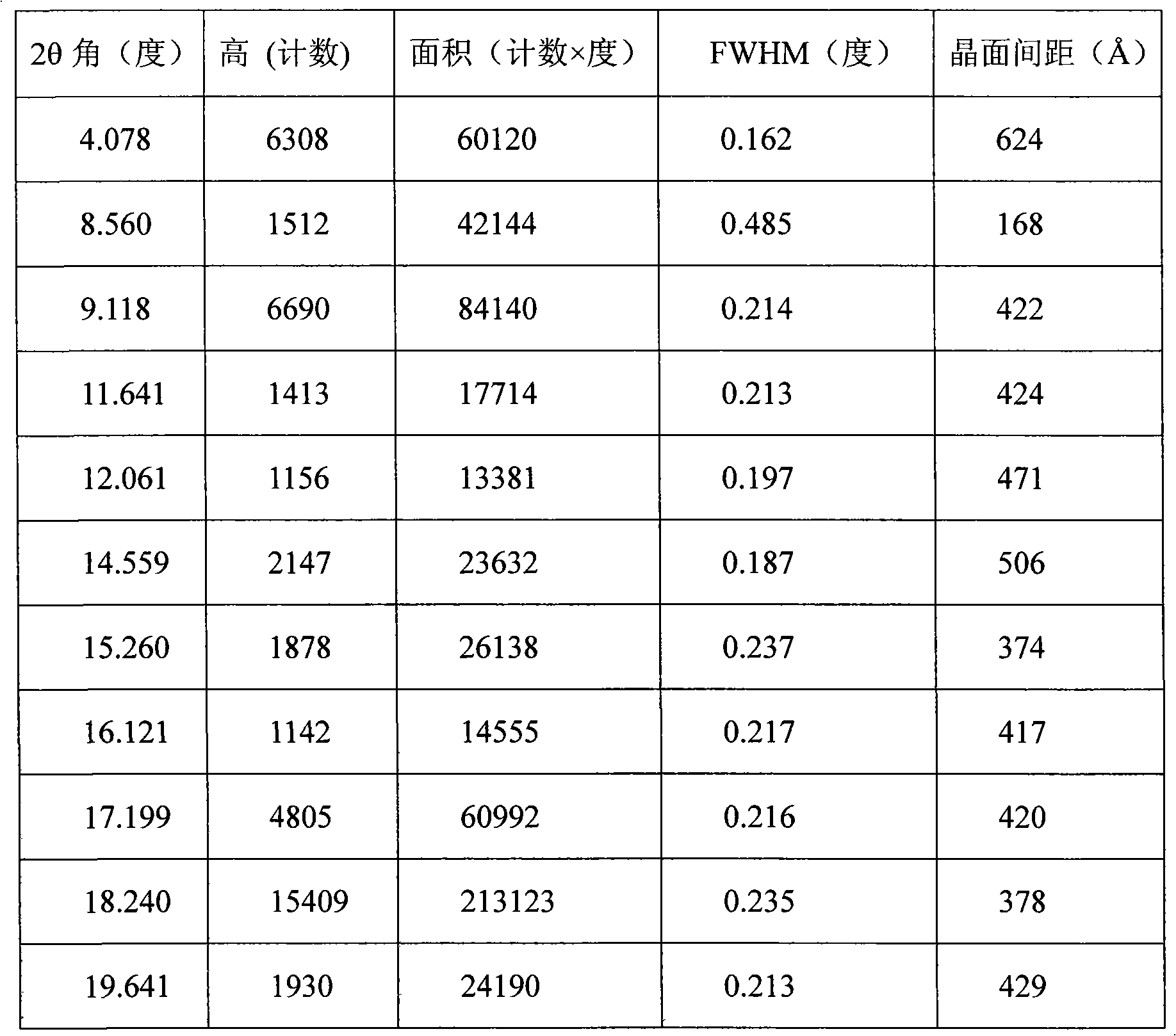
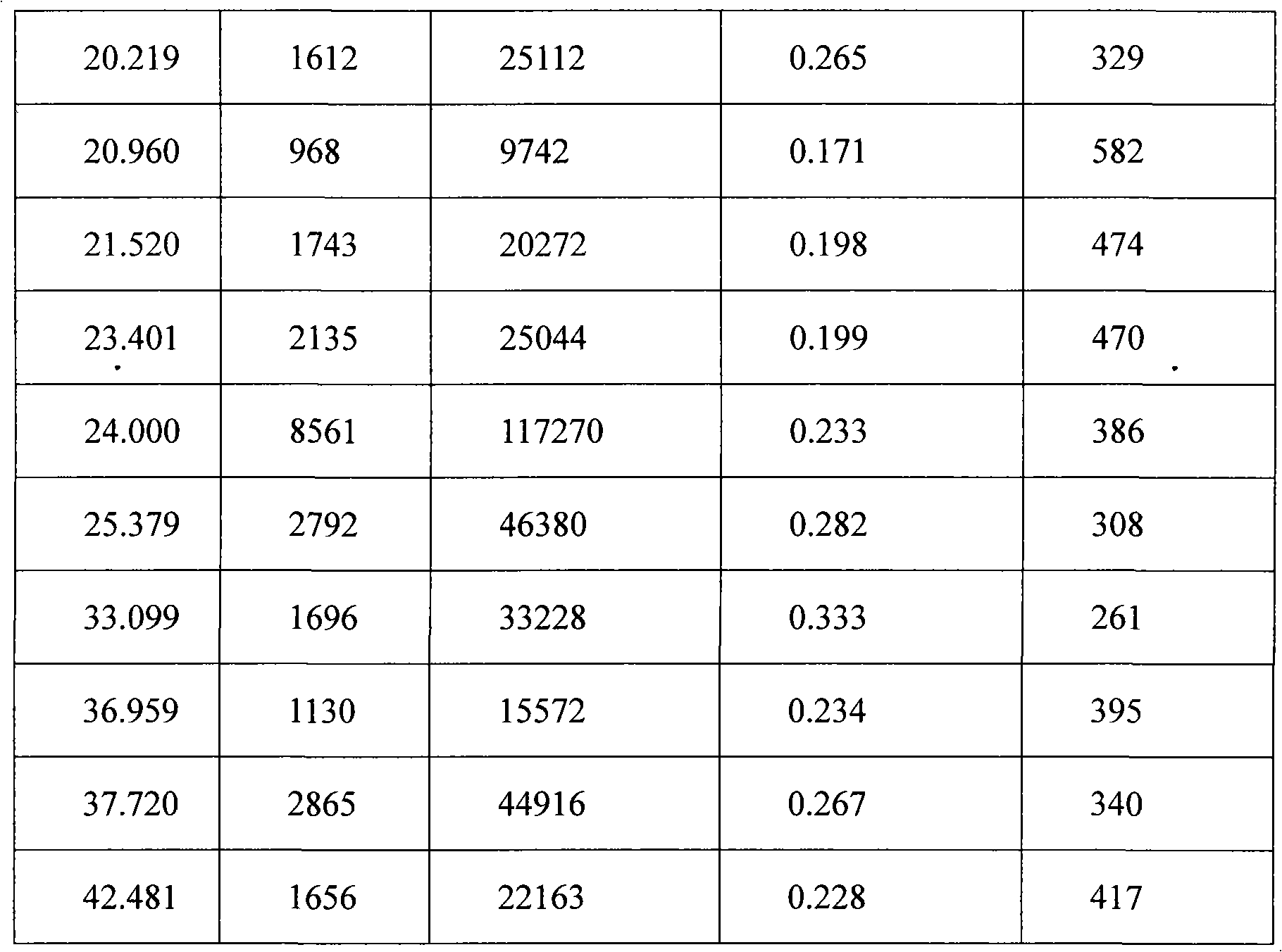
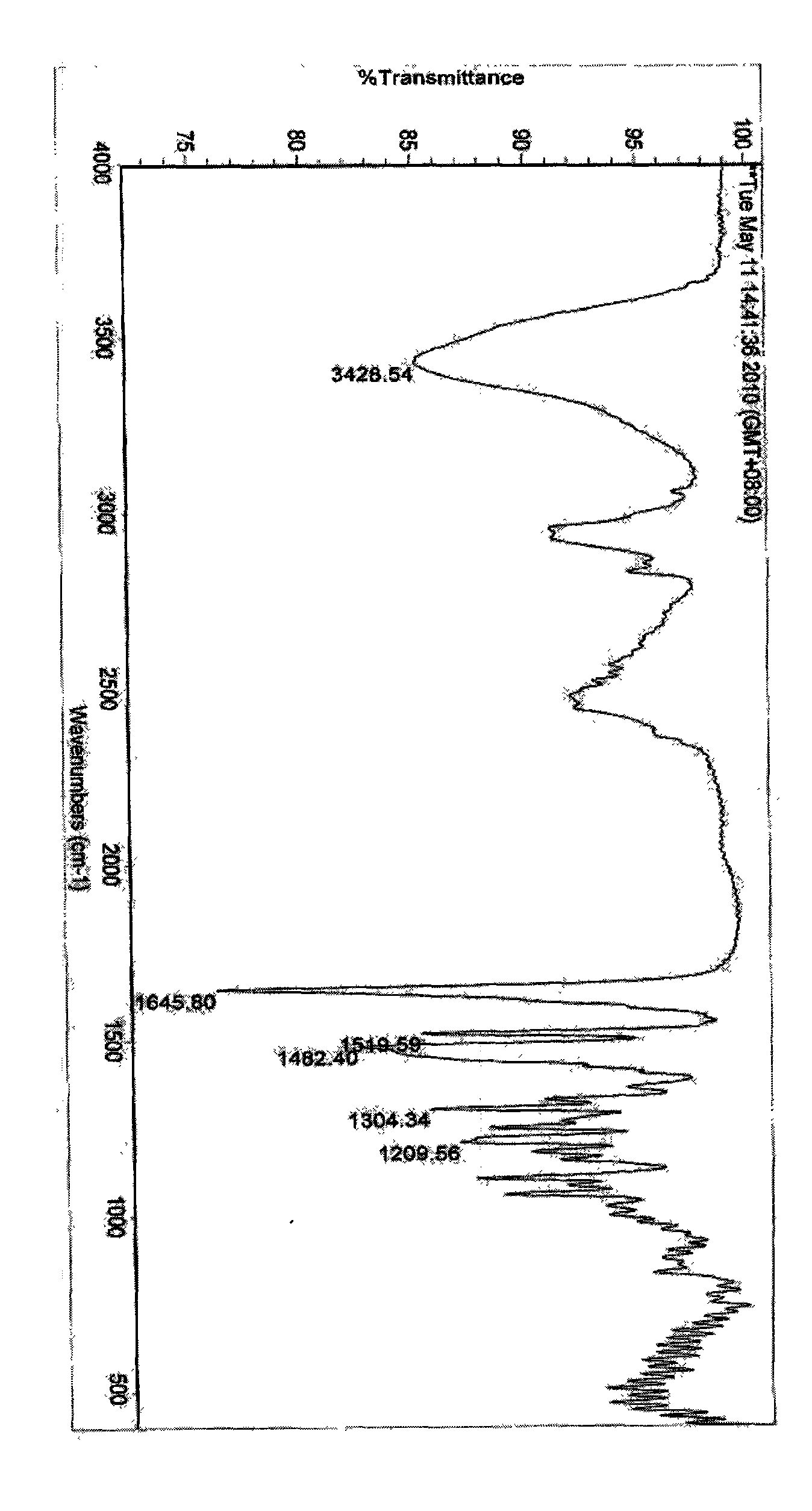
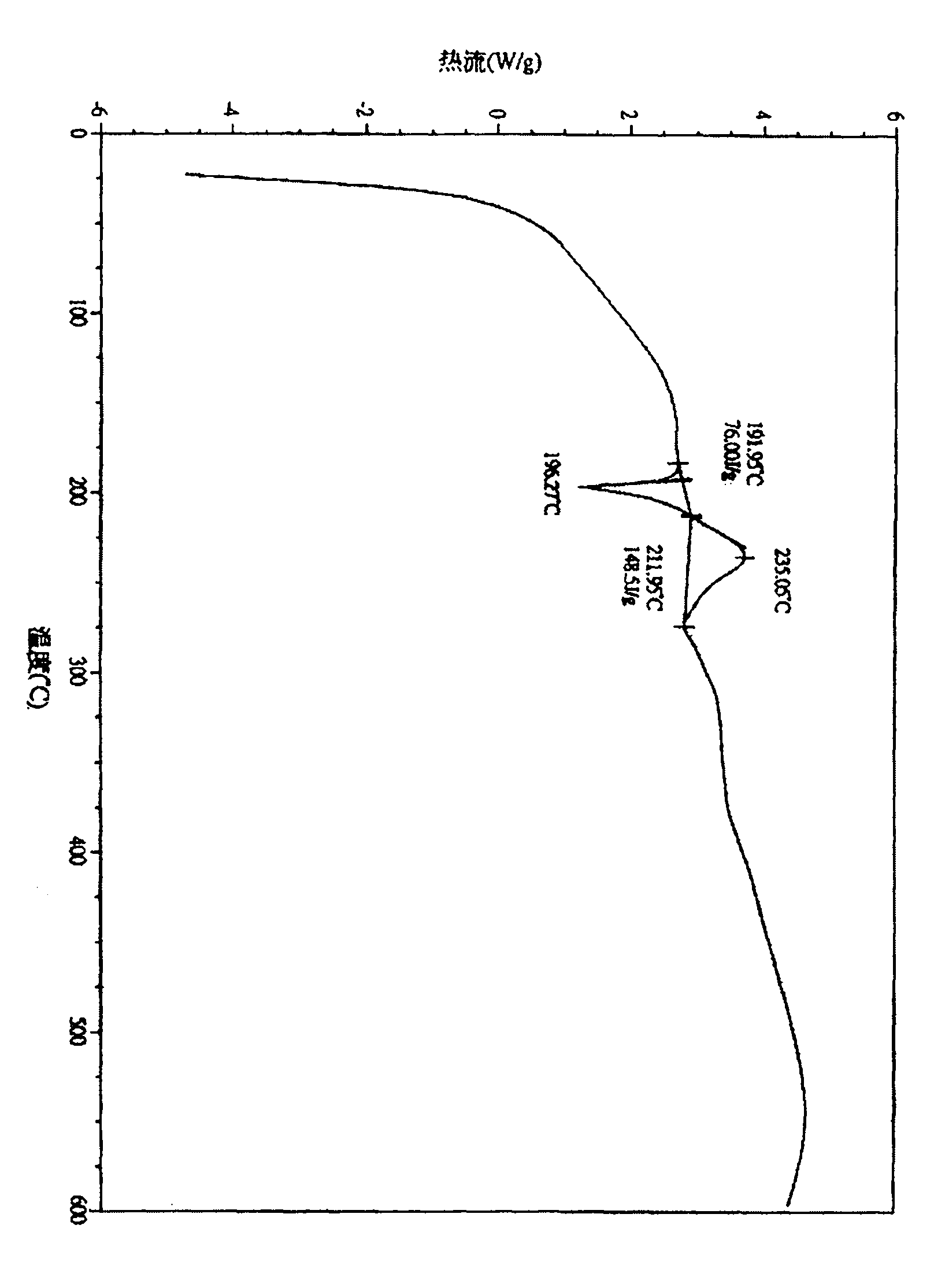
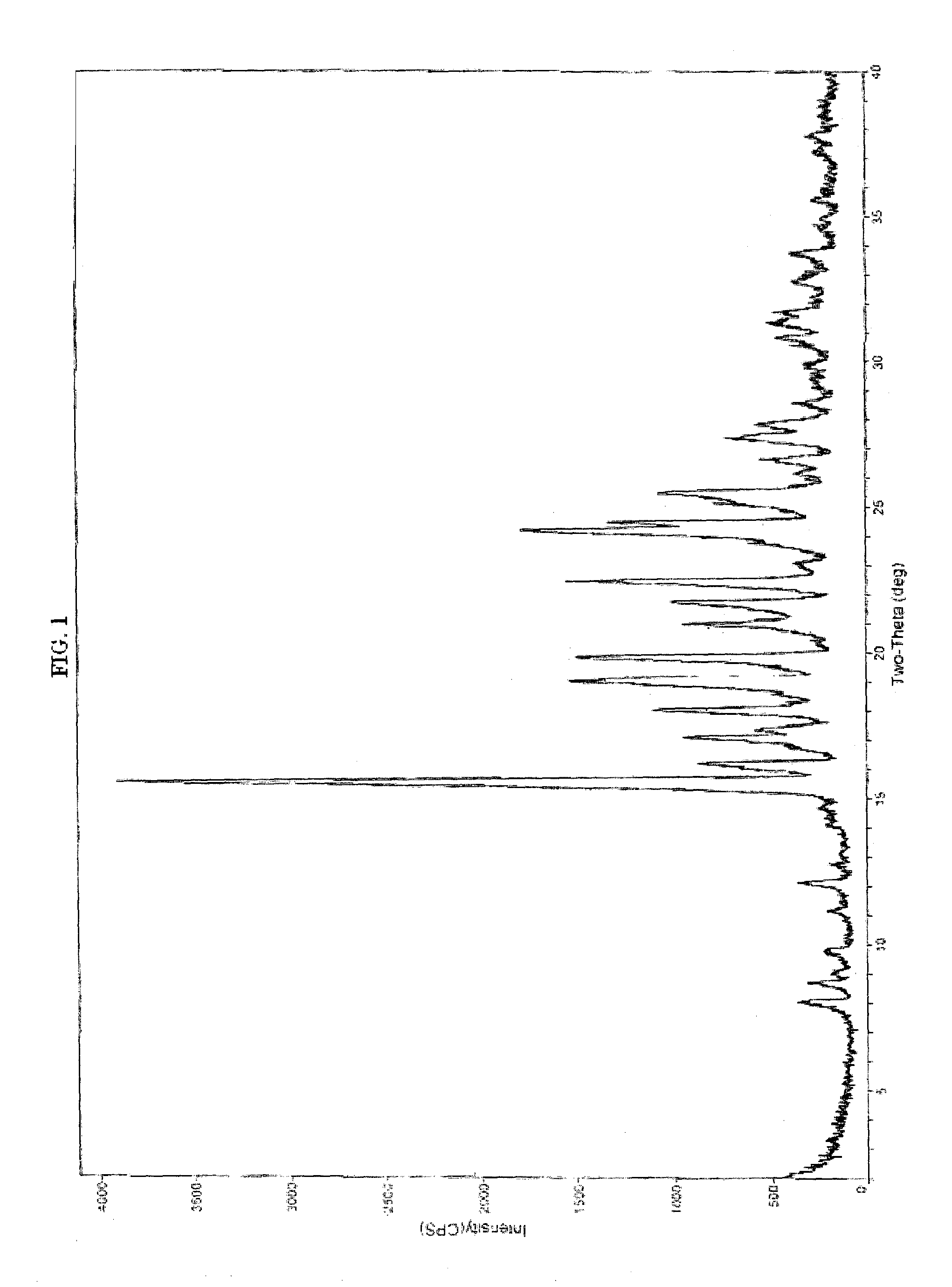
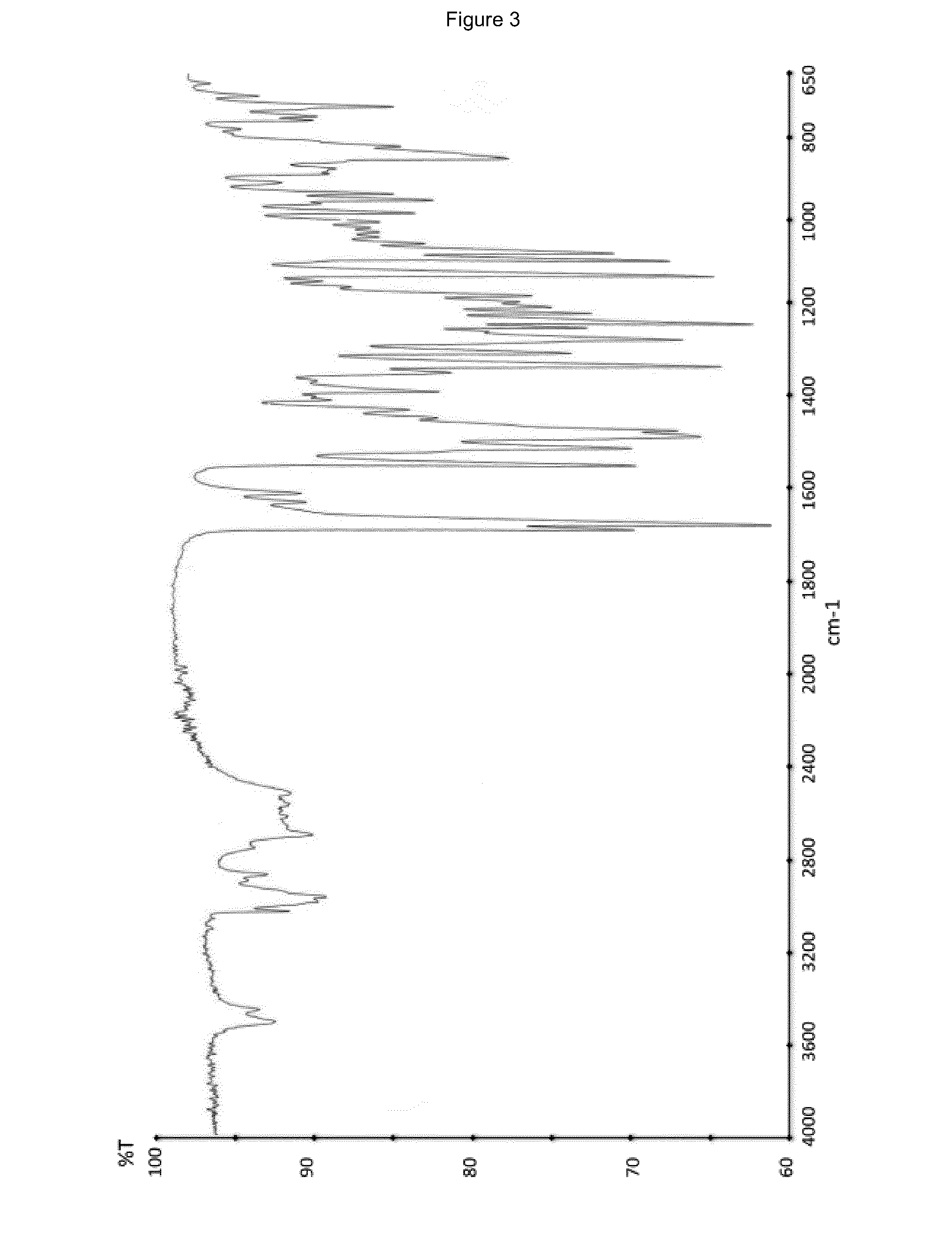
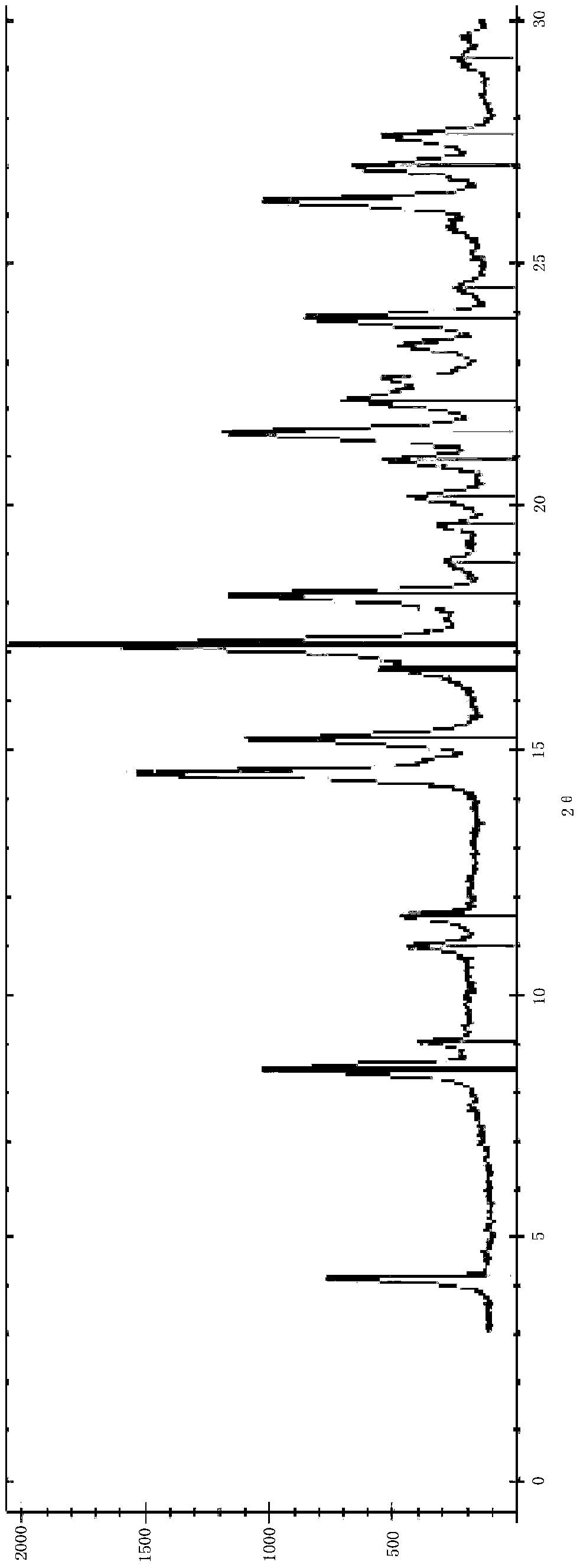
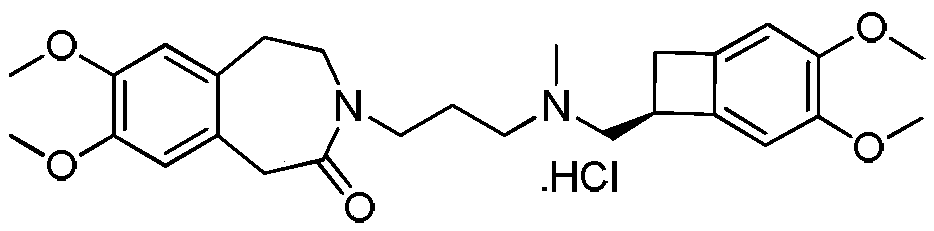
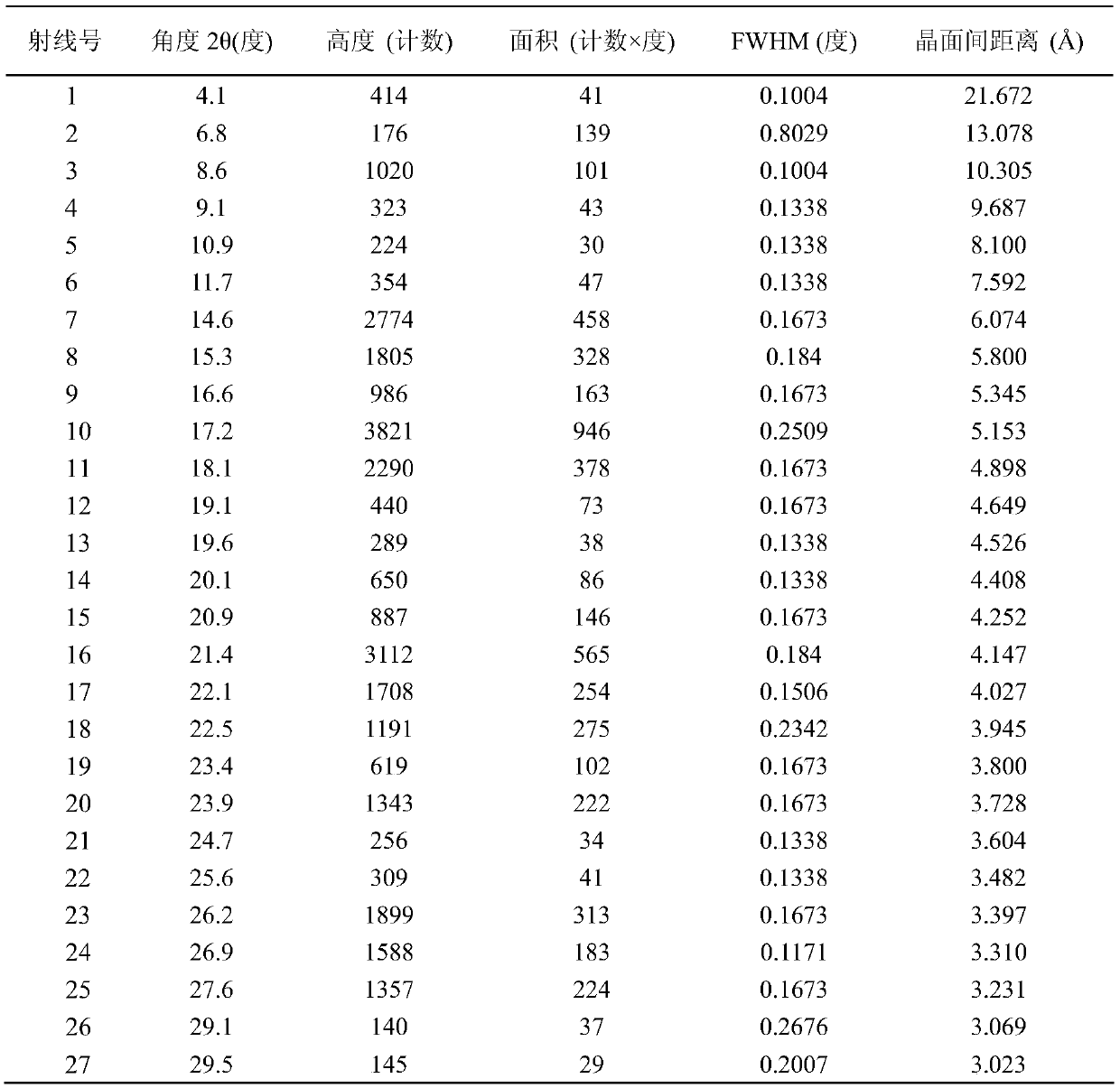
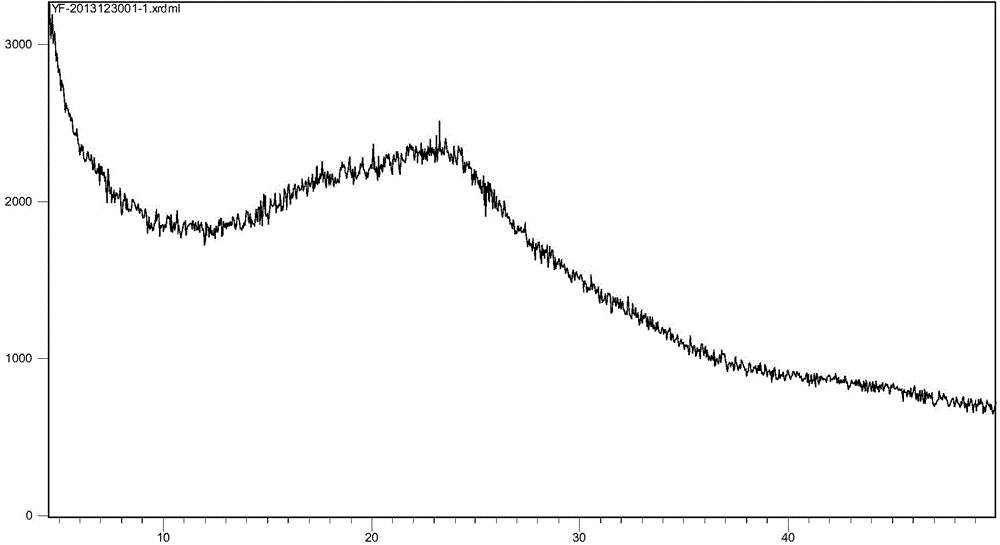
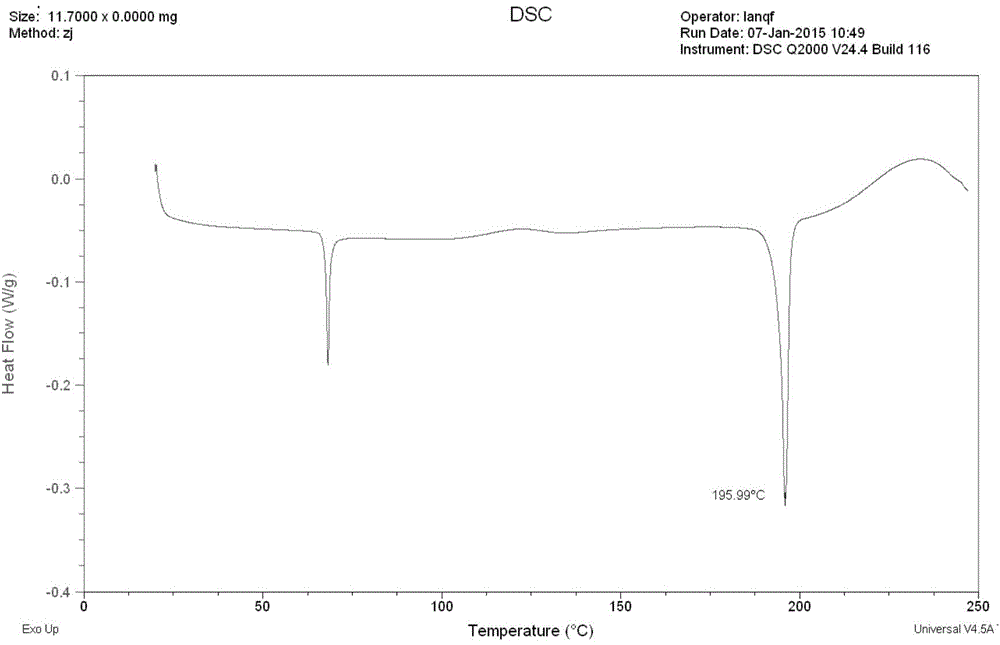
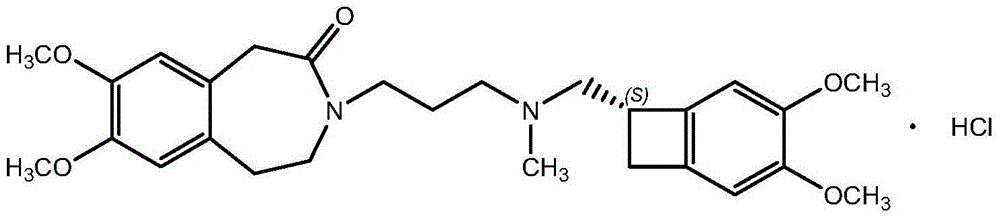
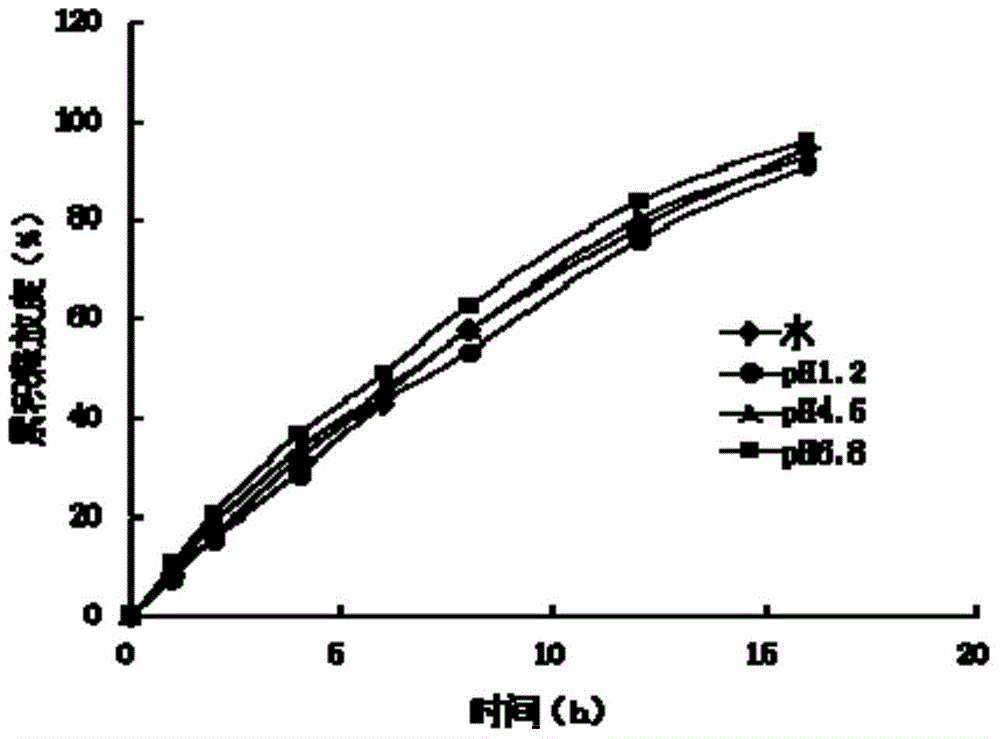
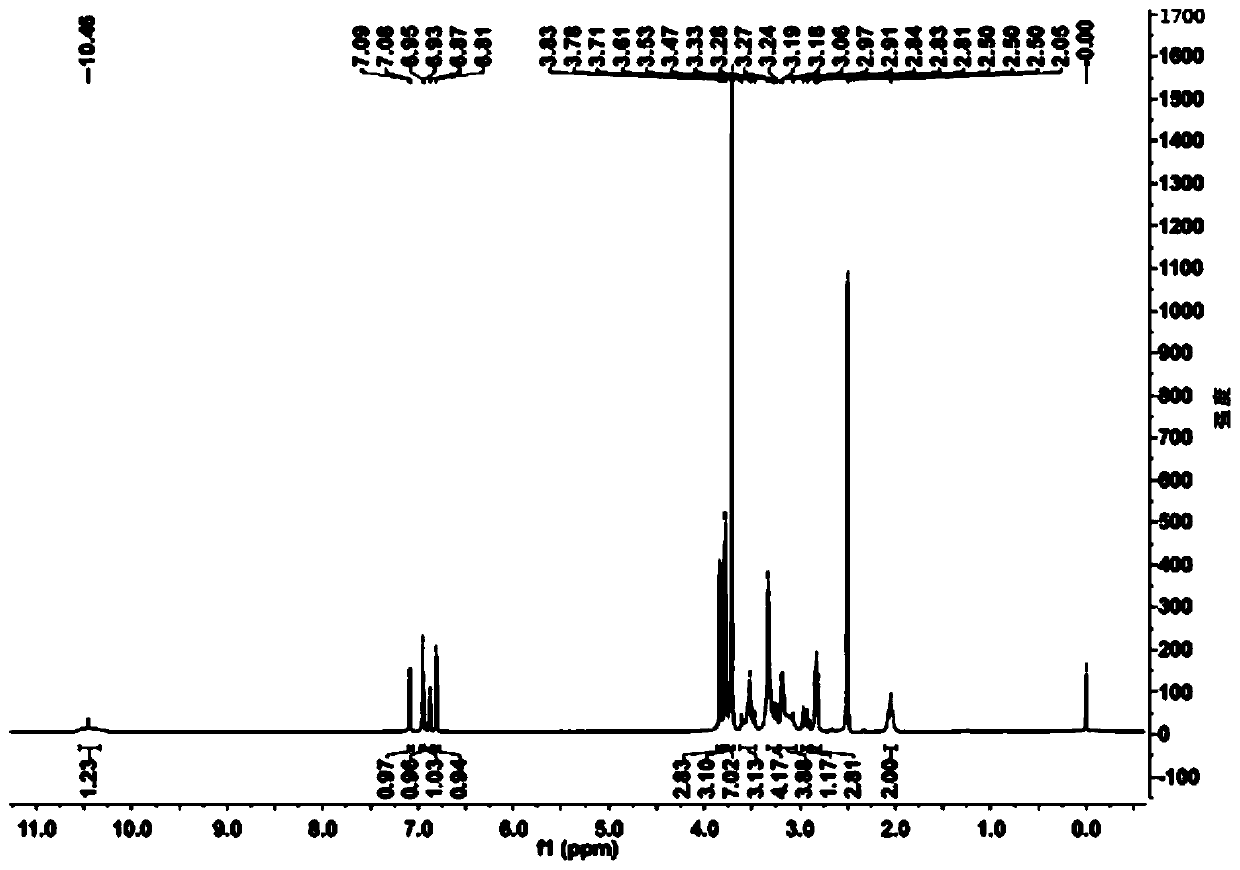
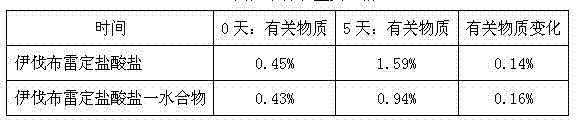
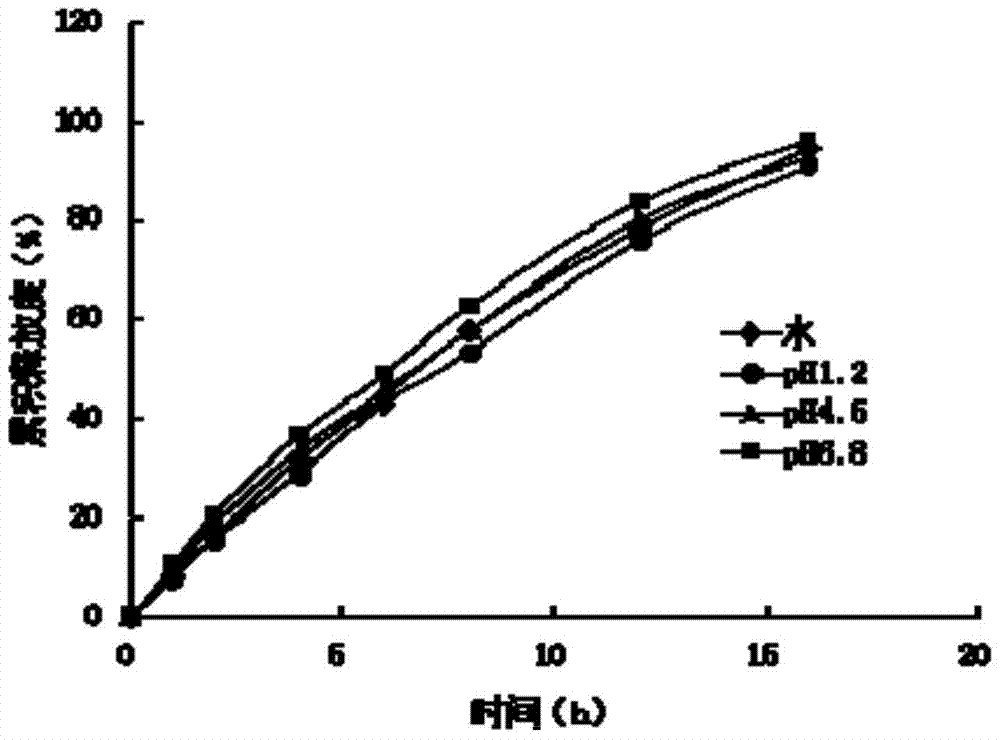
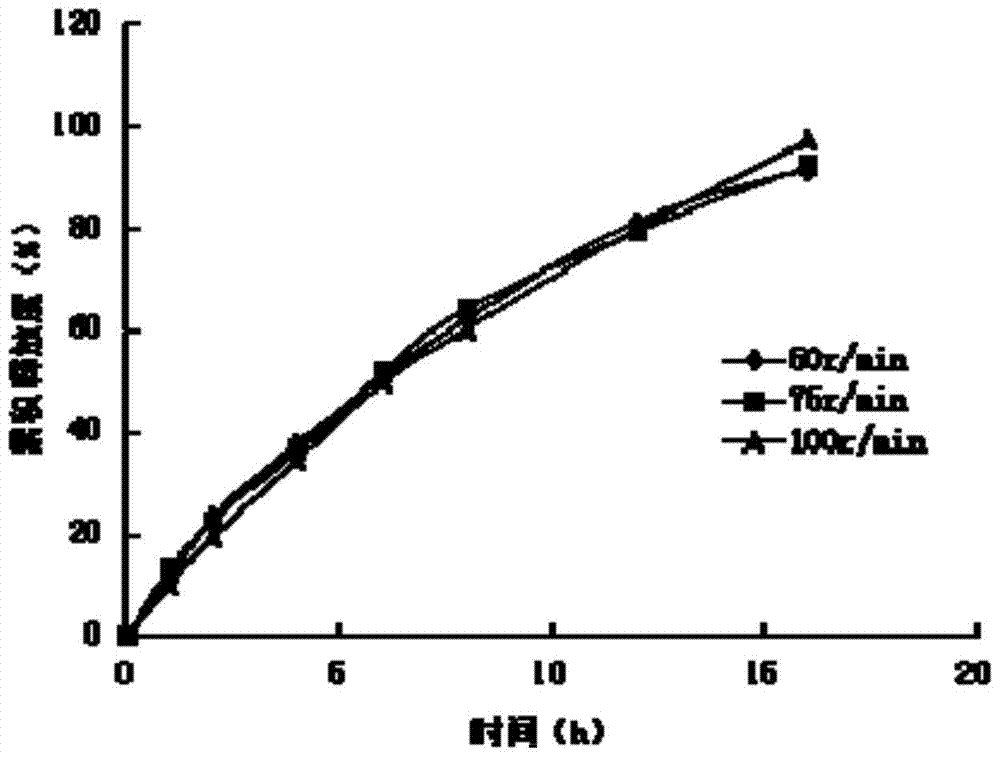
Patents
Literature
75 results about "IVABRADINE HYDROCHLORIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Available as ivabradine hydrochloride; dosage expressed in terms of ivabradine. 1. Adults Heart Failure Oral. Initially, 5 mg twice daily. 1. Initiate dosage of 2.5 mg twice daily in patients with history of conduction defects, or patients in whom bradycardia could lead to hemodynamic compromise. 1
Process for the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid
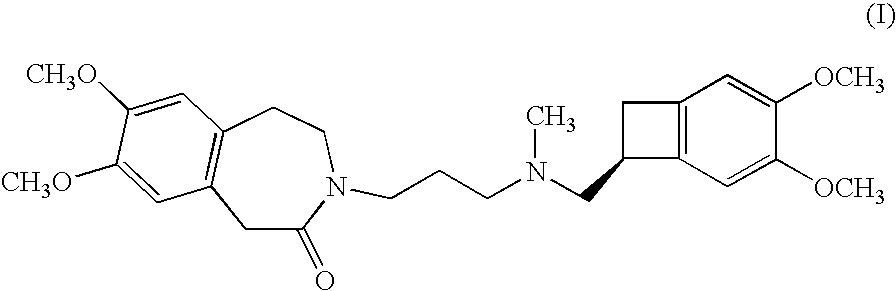
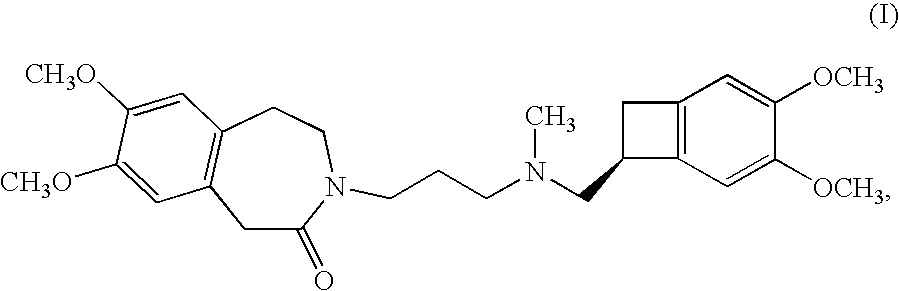
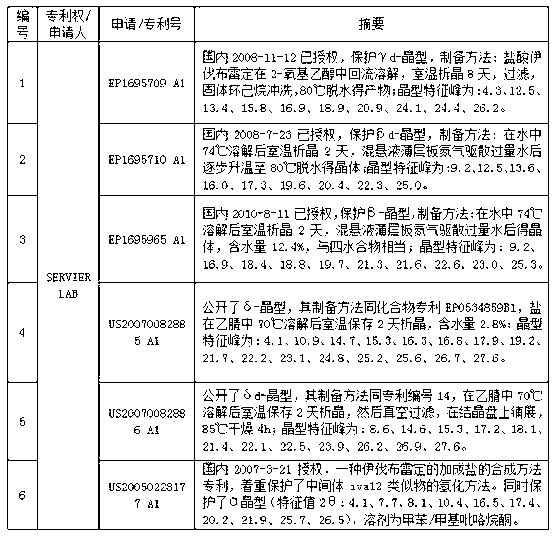
Process for the synthesis of ivabradine of formula (I): addition salts with a pharmaceutically acceptable acid and hydrates thereof. α crystalline form of ivabradine hydrochloride. Medicinal products containing the a crystalline form of ivabradine hydrochloride, which are useful as bradycardics.
Owner:LES LAB SERVIER
Delta d-crystalline form of ivabradine hydrochloride, a process for its preparation and pharmaceutical compositions containing it
InactiveCN1948293AOrganic chemistry methodsCardiovascular disorderIVABRADINE HYDROCHLORIDEMedicinal chemistry
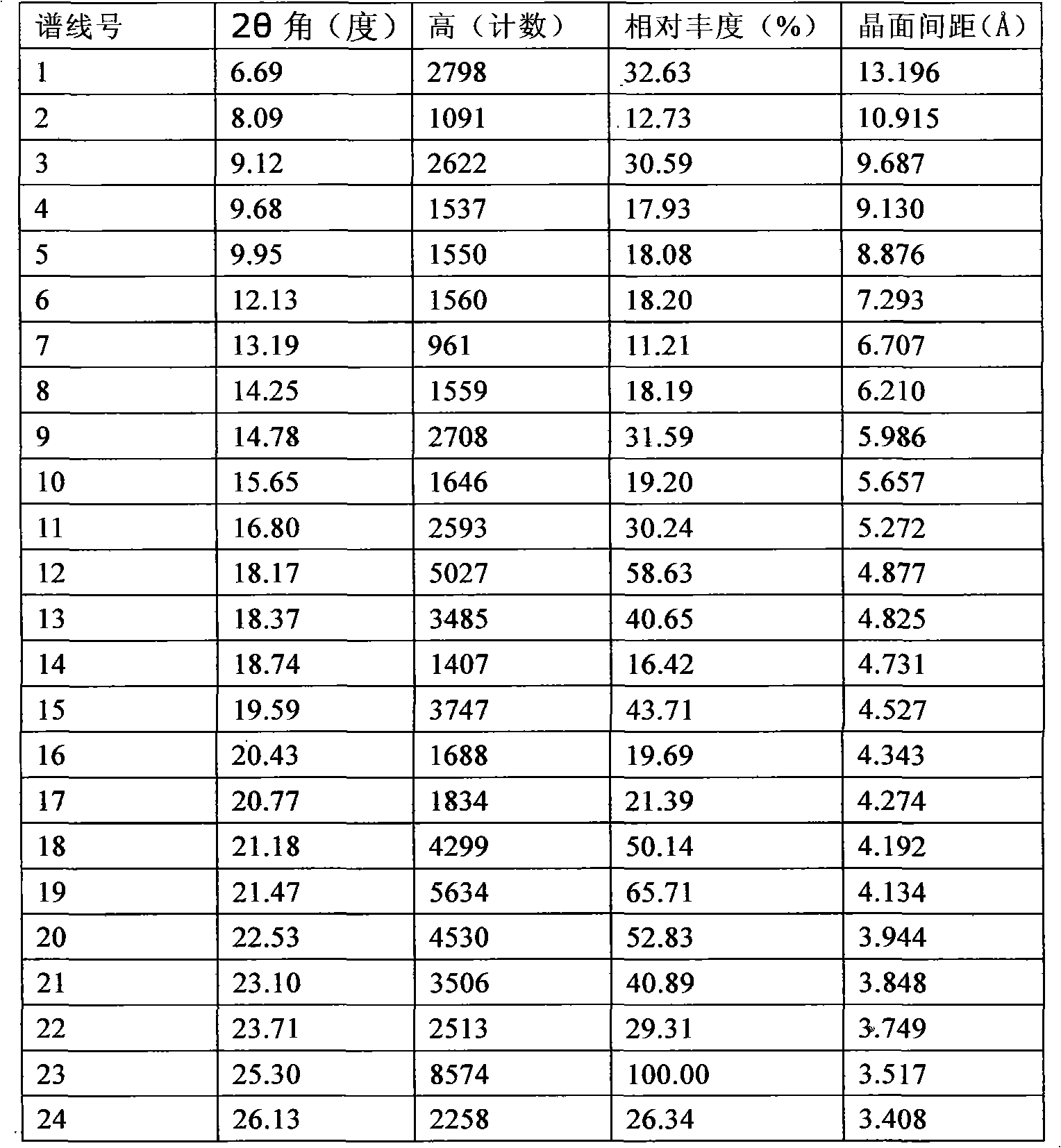
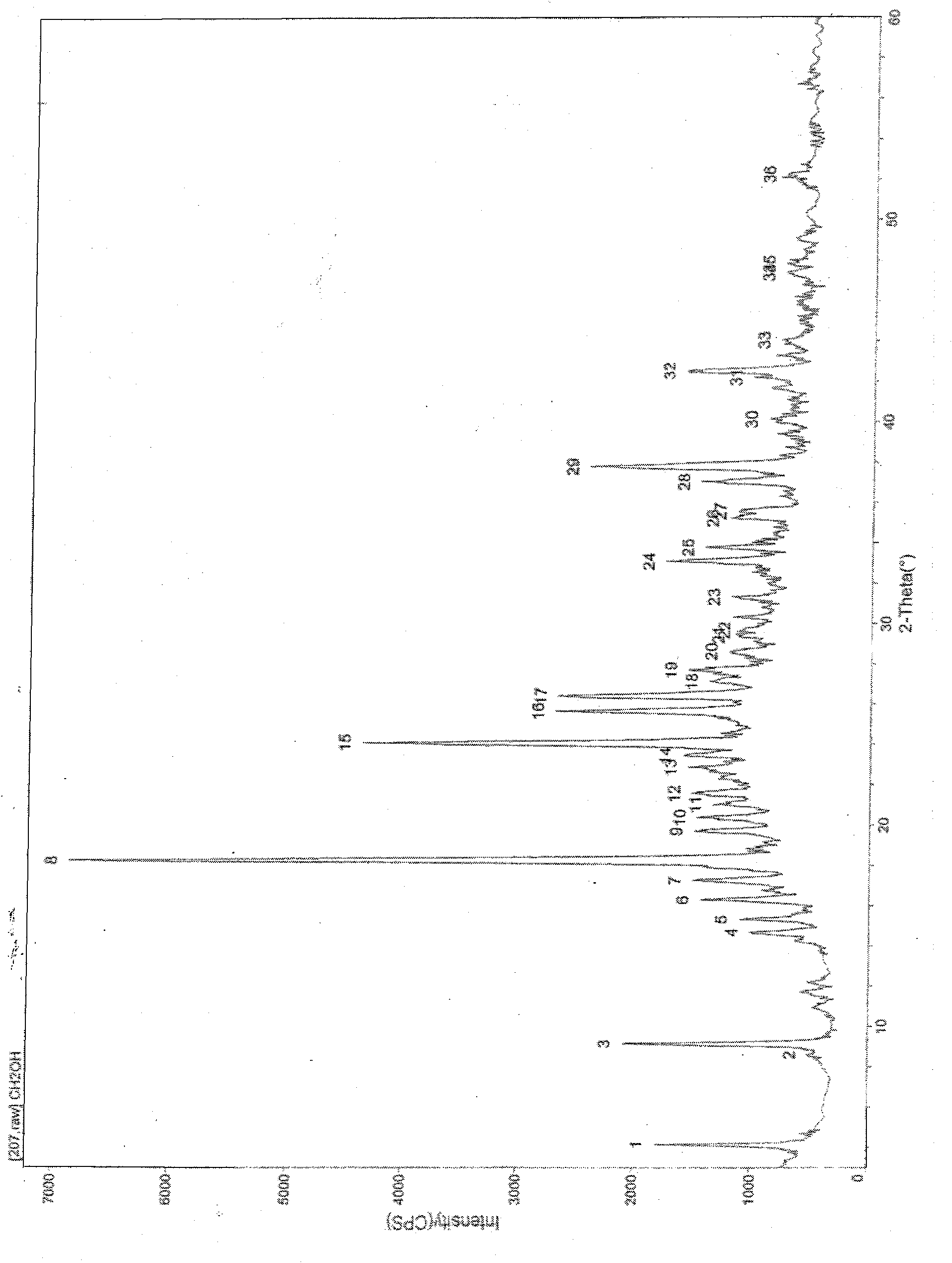
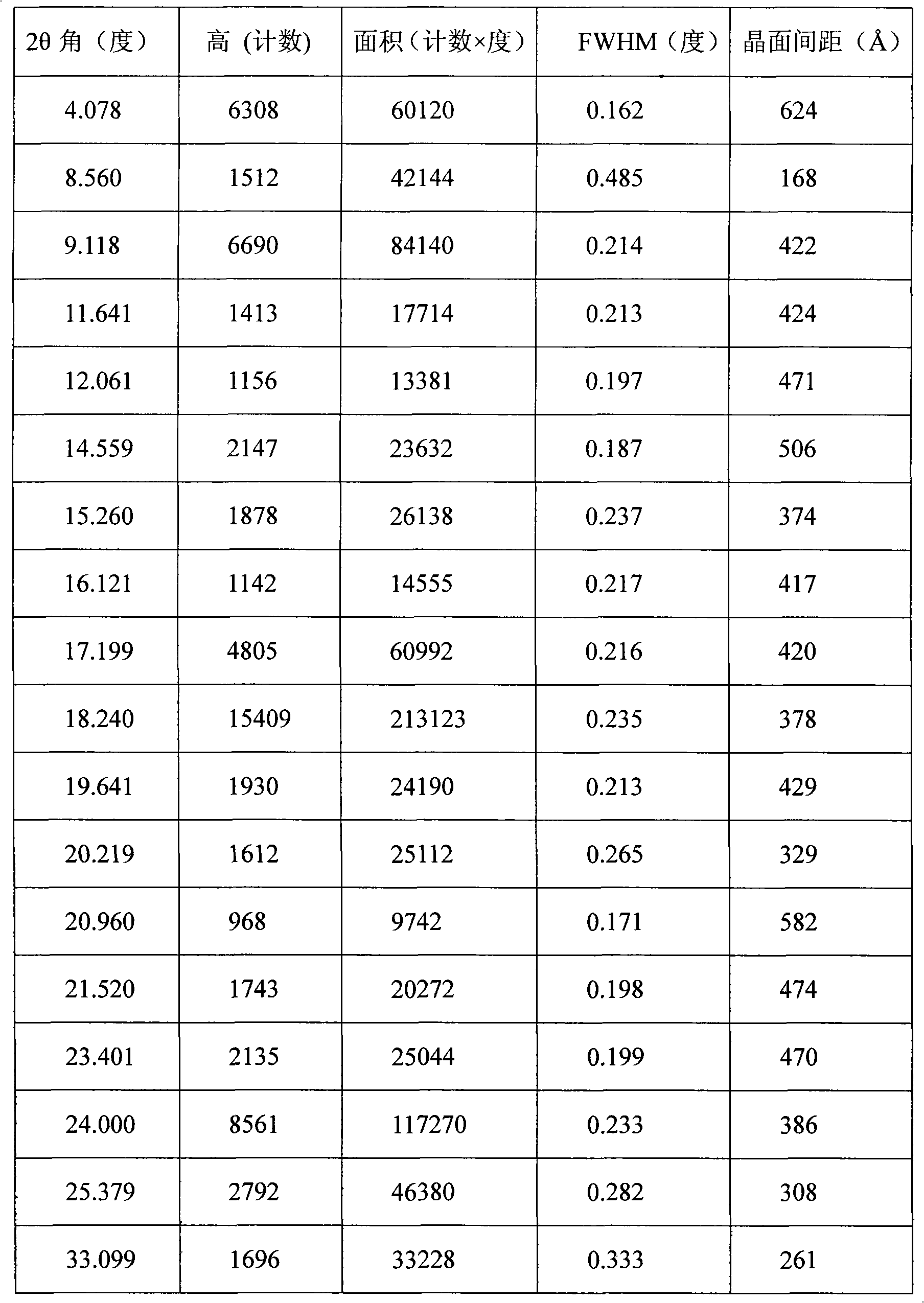
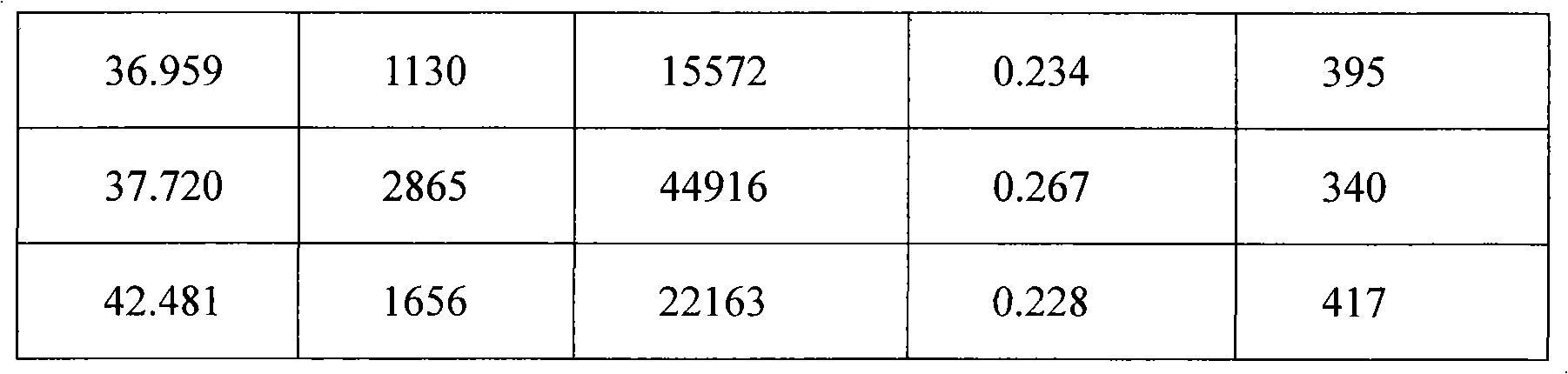
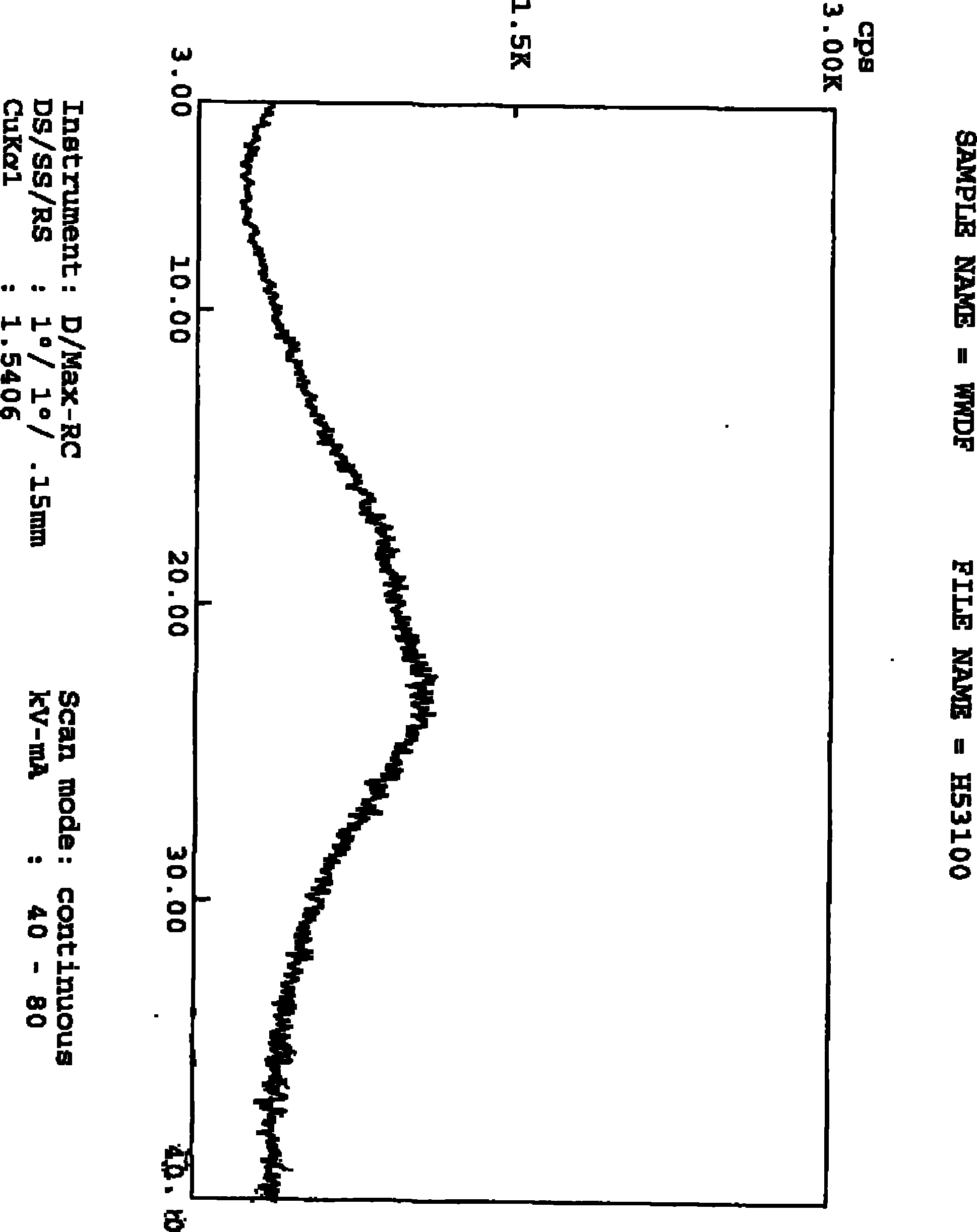
The invention relates to the dd crystalline form of ivabradine hydrochloride having general formula (I), which is characterised by its X-ray diffraction pattern on powder. The invention is suitable for medicaments.
Owner:SERVIER LAB
Delta,-crystalline form of ivabradine hydrochloride, a process for its preparation and pharmaceutical compositions containing it
InactiveCN1948292AOrganic chemistry methodsCardiovascular disorderIVABRADINE HYDROCHLORIDEMedicinal chemistry
The invention relates to the d crystalline form of ivabradine hydrochloride having general formula (I), which is characterised by its X-ray diffraction pattern on powder. The invention is suitable for medicaments.
Owner:SERVIER LAB
Omega-crystal form, preparation method and medicine composite of ivabradine hydrochloride
InactiveCN101805289AImprove humidity stabilityPrevent anginaOrganic chemistryCardiovascular disorderIVABRADINE HYDROCHLORIDEOrganic solvent
The invention discloses a new crystal form omega-crystal form of ivabradine hydrochloride and preparation method thereof. The mixture of the ivabradine hydrochloride and water is heated and dissolved completely and then is frozen and dried to obtain delta-crystal form crystal. The crystal form has stable temperature and humidity, does not contain any organic solvent and moisture and is applicable to preparation technical process and a long period of storage. The medicine composite taking the crystal form composite as active ingredient can be used to treat or prevent angina, myocardial infarction and relative arhythmicity.
Owner:成都威克药业有限责任公司
Process for the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid
Process for the synthesis of ivabradine of formula (I):addition salts with a pharmaceutically acceptable acid and hydrates thereof.α crystalline form of ivabradine hydrochloride.Medicinal products containing the a crystalline form of ivabradine hydrochloride, which are useful as bradycardics.
Owner:LES LAB SERVIER
Amorphous ivabradine hydrochloride
InactiveCN101597261AOrganic chemistryHeterocyclic compound active ingredientsIVABRADINE HYDROCHLORIDEChemistry
The invention discloses amorphous ivabradine hydrochloride and a preparation method thereof. The amorphous ivabradine hydrochloride is detected by means of powder X-ray diffraction and at least 80 percent of the amorphous ivabradine hydrochloride exists in an amorphous form. The preparation method of the amorphous ivabradine hydrochloride comprises a step of desolvating a solution containing the ivabradine hydrochloride to obtain an amorphous solid. In addition, the invention also relates to a medicament composite containing the amorphous ivabradine hydrochloride.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
New benzocyclobutane, preparation method thereof and application thereof
InactiveCN101671265AEasy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationIVABRADINE HYDROCHLORIDECombinatorial chemistry
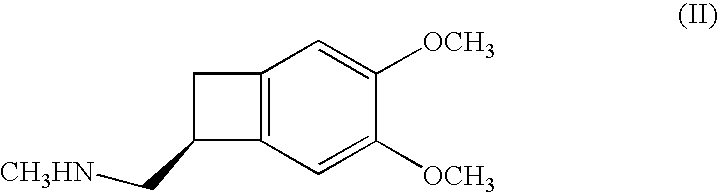
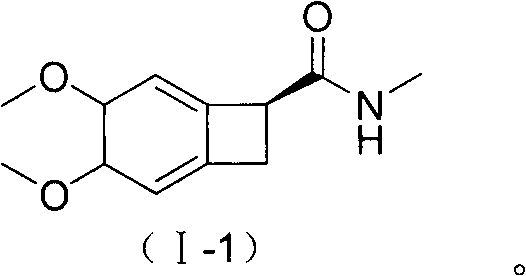
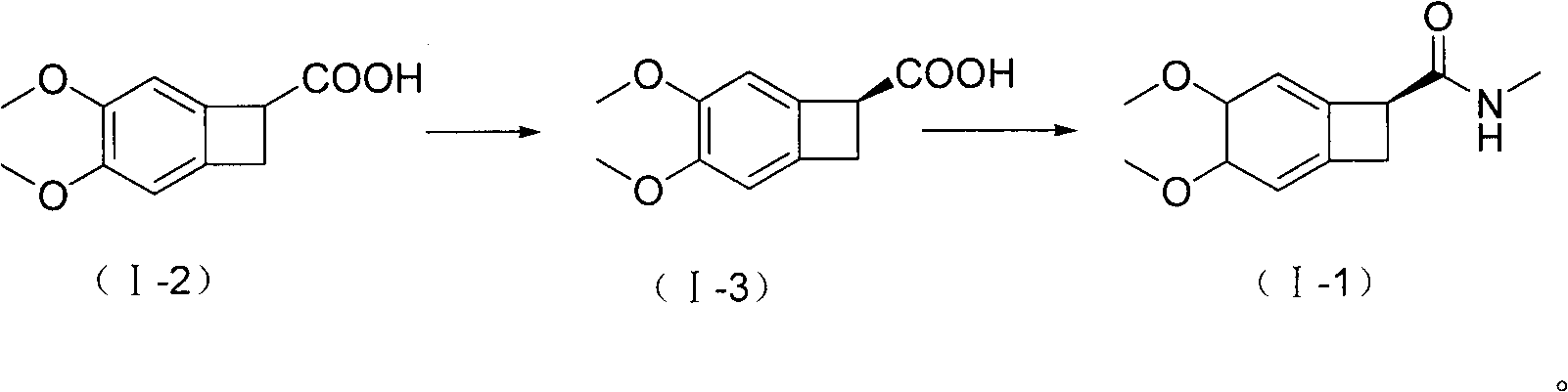
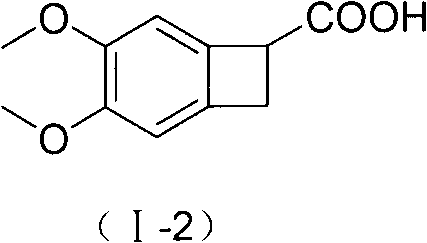
The invention relates to new benzocyclobutane, a preparation method thereof and application thereof. The invention provides a new preparation method for (1S)-4, 5-dimethoxy-1-(methyl-amino-methyl)-benzocyclobutane serving as a key intermediate of ivabradine hydrochloride and addition salt thereof, and meanwhile provides a new benzocyclobutane compound which is an intermediate for preparing the (1S)-4, 5-dimethoxy-1-(methyl-amino-methyl)-benzocyclobutane. In addition, the invention also provides a method for preparing the new benzocyclobutane compound, and meanwhile provides a method for splitting an intermediate product during preparing the new benzocyclobutane compound.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
New crystalline form of hydrochloric acid Ivabradine and preparation method thereof
The invention discloses a new crystalline form of hydrochloric acid Ivabradine and a preparation method thereof. A mixture of the hydrochloric acid Ivabradine, methanol and acetone is heated to be completely dissolved, natural stewing is carried out for complete crystallization, and products are collected through filtration so as to obtain the new crystalline form of the hydrochloric acid Ivabradine.
Owner:BEIJING D VENTUREPHARM TECH DEV
Stable Ivabradine crystal and preparation thereof
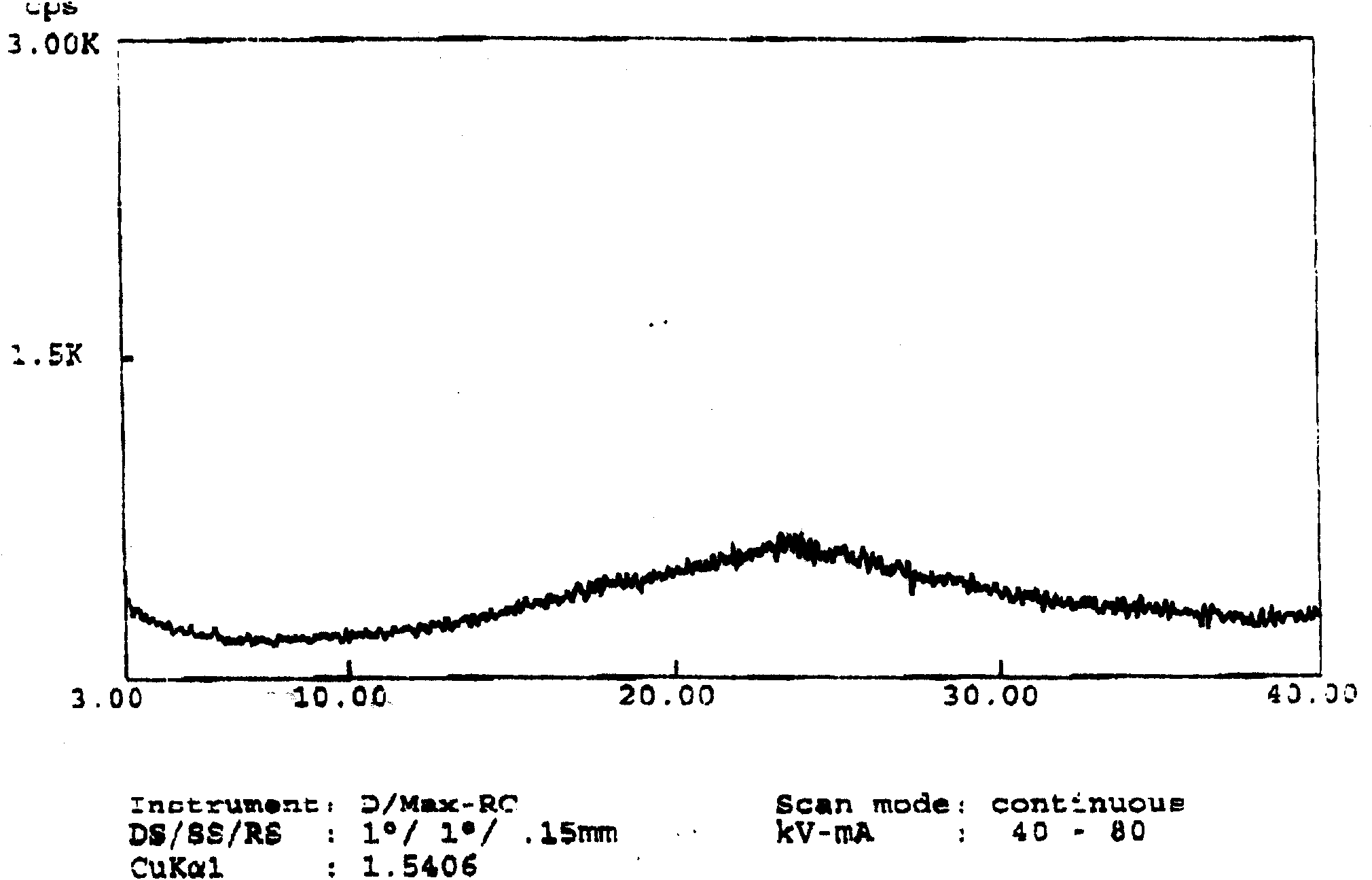
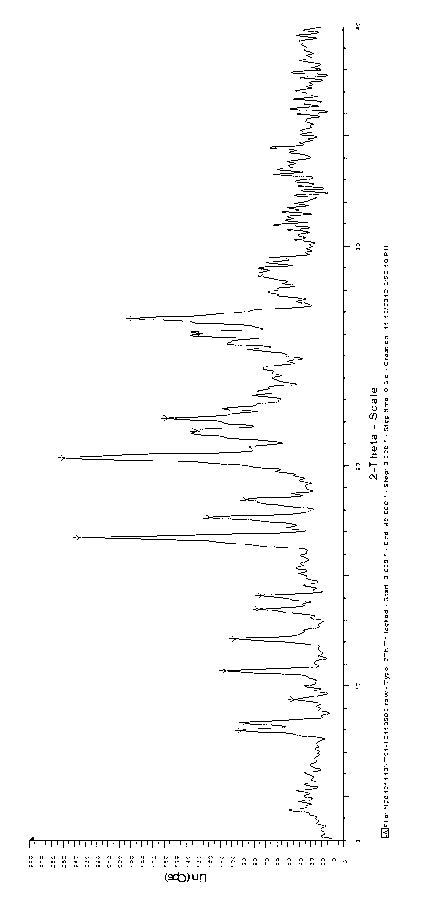
The invention discloses a stable ivabradine hydrochloride crystal (formula I), which is verified by a DCS differential thermal analysis chart, a melting point and an X-ray powder diffraction diagram. By adopting the method, the ivabradine hydrochloride crystal (formula I) prepared by the method is very stable to temperature, illumination and humidity, thus being favorable for long-term storage. The solvent used in the crystal is safe, and is easy to be removed; therefore, the stable ivabradine hydrochloride crystal is applicable to industrialized production.
Owner:UTOPHARM SHANGHAI +1
Stable hydrochloric acid Ivabradine II crystal form and preparation method
ActiveCN103183639AEasy to preparePromote environmental protectionOrganic chemistrySpace groupDecomposition
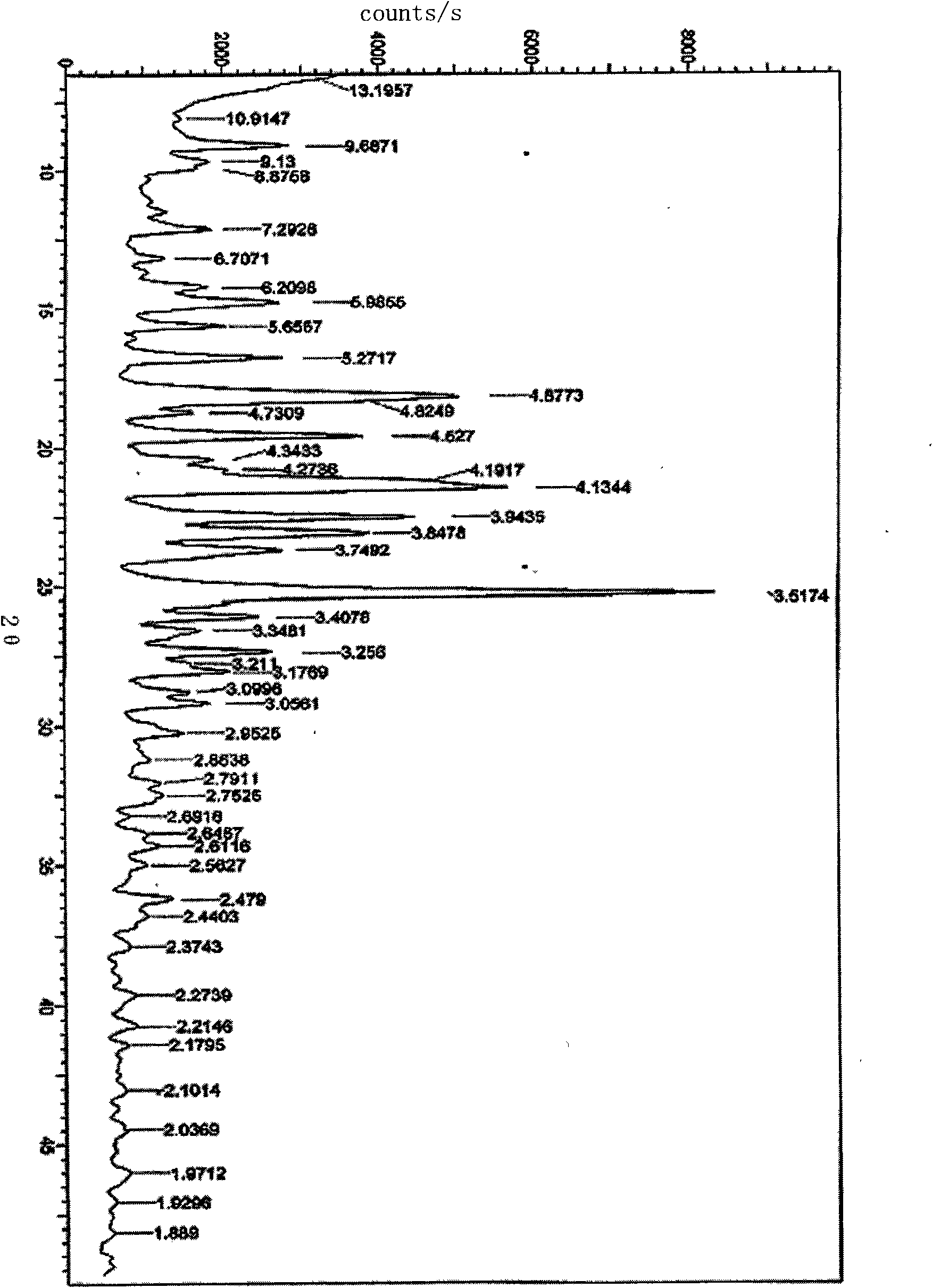
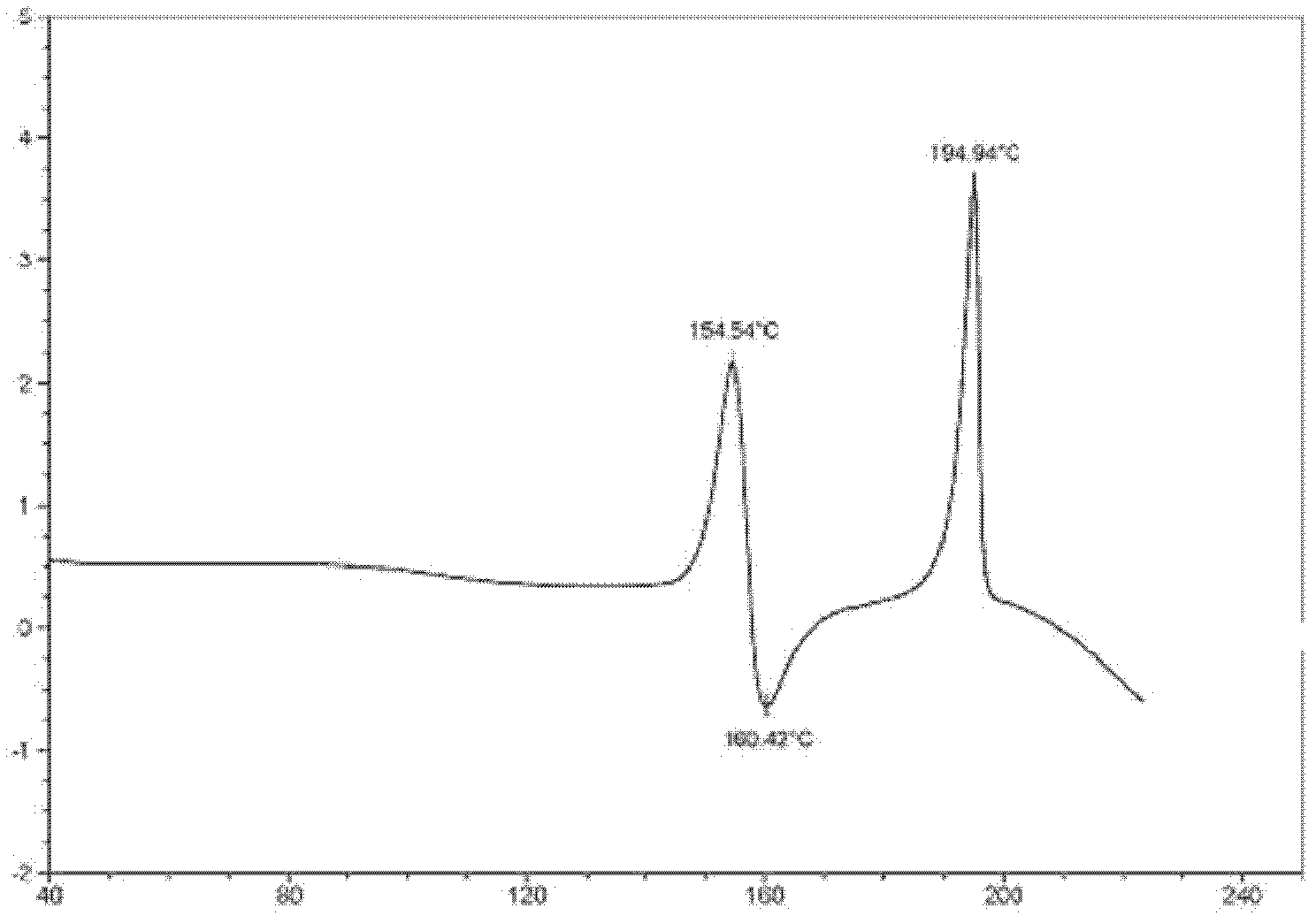
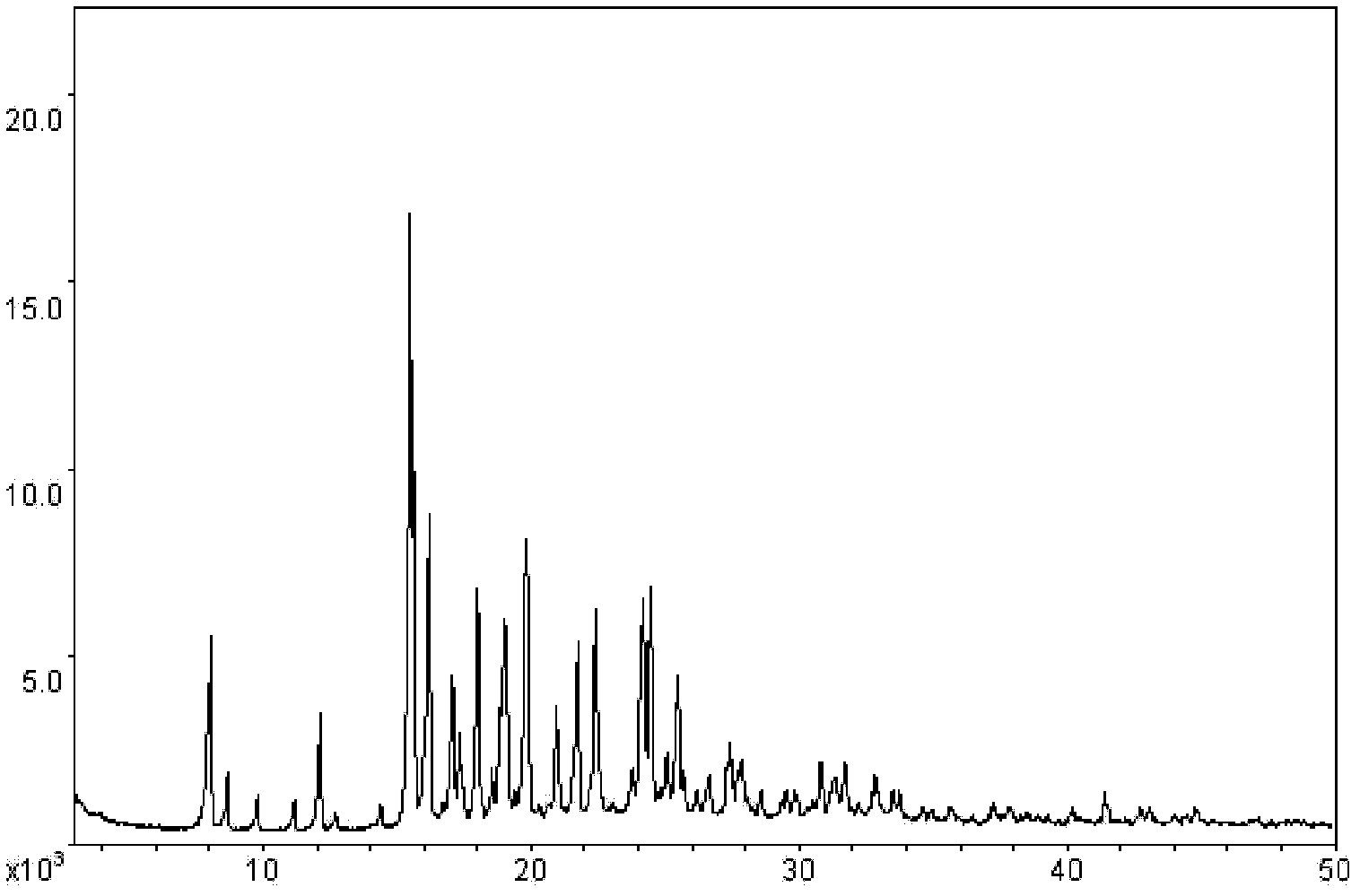
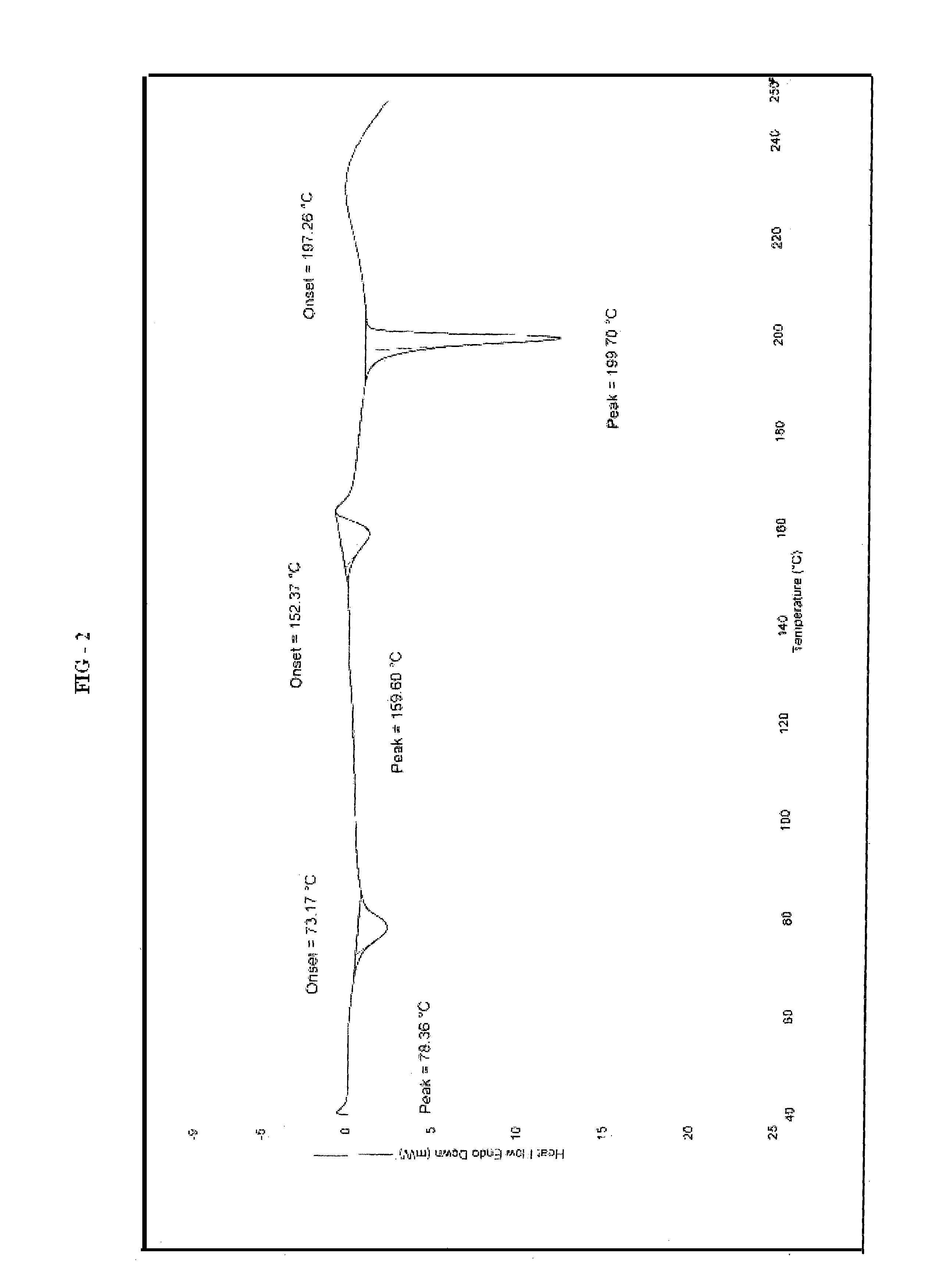
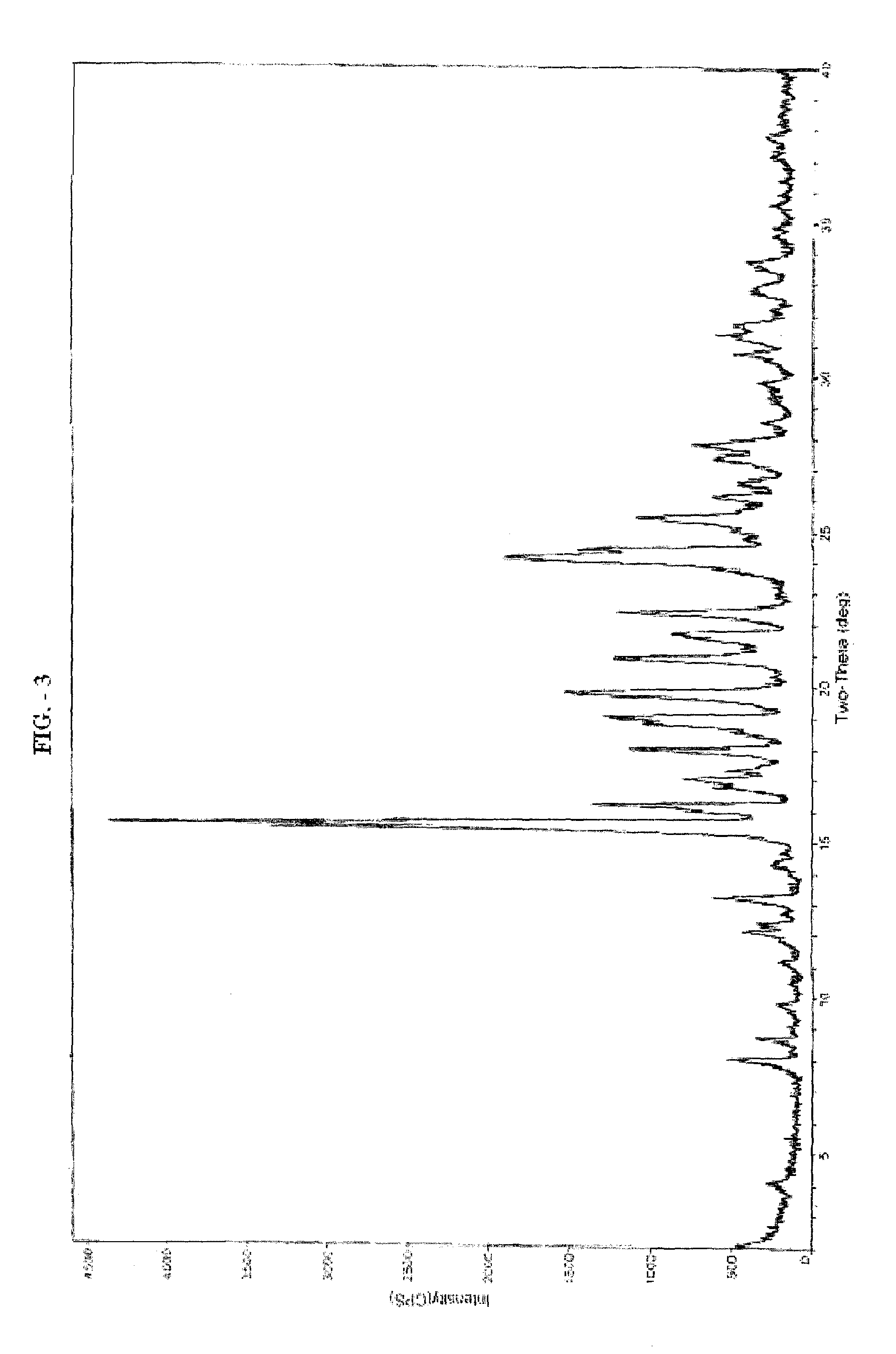
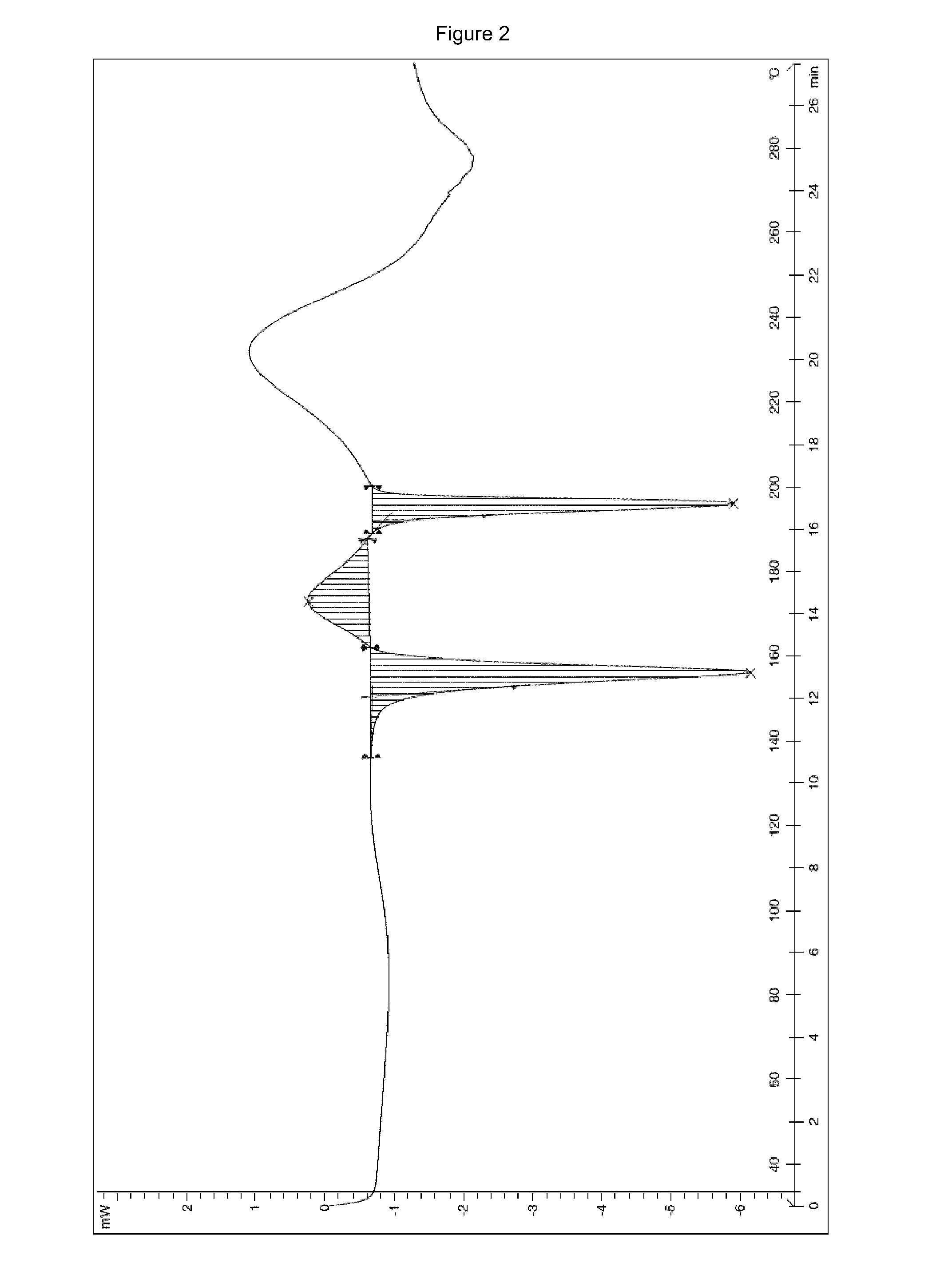
The invention provides a stable hydrochloric acid Ivabradine II crystal form, a thermogravimetry spectrogram shows that each part of hydrochloric acid Ivabradine molecule contains 0.5 parts of crystal water. The differential scanning calorimetry (DSC) spectrum diagram shows that a large endothermic peak is generated at -154 DEG C, a heat release peak is generated at -160 DEG C, wherein the fusion decomposition temperature is 194 DEG C (summit value), wherein the hydrochloric acid Ivabradine II crystal form is a monoclinic system, its space group is P21, the cell parameter comprises: a=5.48740(10)Angstrom, b=43.4767(7)Angstrom, c=11.4892(2)Angstrom, beta= 98.144 (2) degrees, and a crystal cell volume is 2713.38(8)Angstrom<3>. A characteristic diffraction spectral line of its X-powder diffraction describes hydrochloric acid Ivabradine new crystal form as II crystal form. The Ivabradine new crystal form enables difficult water absorption and deliquescence, and has the advantages of good stability and convenient storage. The preparation method is simple and easy to be carried out, a solvent with high boiling point and large toxicity is not used, the preparation method is in favor of environmental protection, is suitable for industrial production, and has large application value.
Owner:ZHEJIANG JINGXIN PHARMA +1
Hydrochloric acid Ivabradine solid pharmaceutical composition and method for preparing the same
InactiveCN101152155AExpand the application range of dosage formsImprove bioavailabilityPill deliveryCapsule deliveryIVABRADINE HYDROCHLORIDEAngina
The invention discloses an oral drug combination and the preparation method. The combination of the invention contains Ivabradine Hydrochloride and medically acceptable vector and exists in forms of tablets, capsules or particles. The preparation process of the combination is simple and is suitable for industrial production. And the combination is used to cure cardiovascular diseases including angina pectoris.
Owner:BEIJING D VENTUREPHARM TECH DEV
Novel method for preparing amorphous Ivabradine hydrochloride
ActiveCN102050784AHigh yieldIncrease productivityOrganic chemistryNon solventIVABRADINE HYDROCHLORIDE
The invention provides a novel method for preparing amorphous Ivabradine hydrochloride, which comprises the following steps: firstly, Ivabradine hydrochloride is dissolved in a solvent to form a solution, and secondly, the solution is added into a non-solvent, a mixed solution is obtained through stirring, and amorphous Ivabradine hydrochloride is obtained after cooling. The method has the advantage that the traditional two-step amorphous preparation method (crystal type high-quality product is produced at first and then amorphous is prepared through a concentration method) is integrated into one step, so as to achieve high yield and greatly improve the production efficiency.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA +1
Ivabradine hydrochloride tablet and preparation method thereof
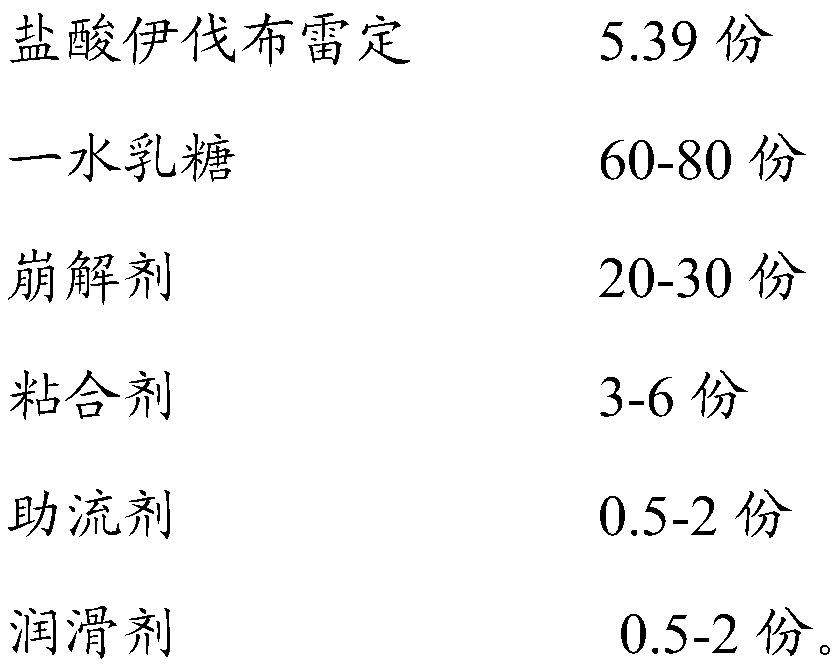
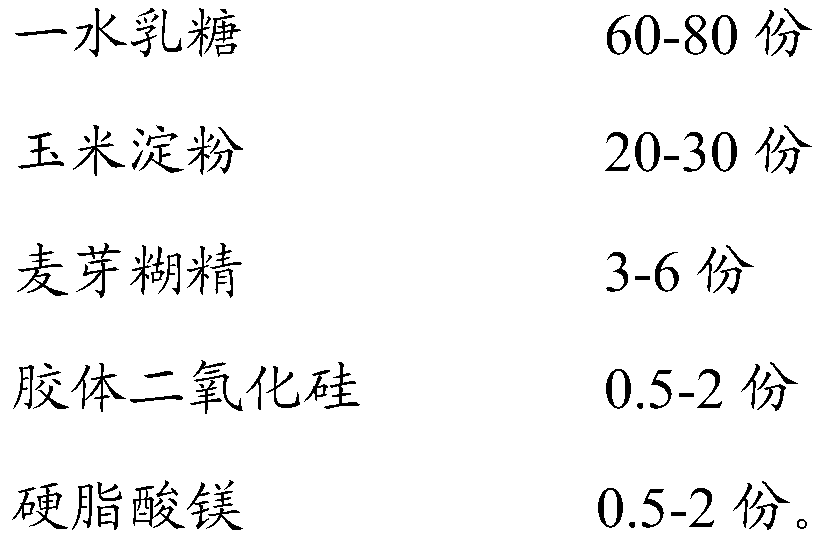
InactiveCN106265582AImprove compliancePharmaceutical non-active ingredientsDrageesLACTOSE MONOHYDRATEIVABRADINE HYDROCHLORIDE
The invention belongs to the technical field of medicine preparation, and relates to an ivabradine hydrochloride tablet and a preparation process thereof. A mark is formed in the ivabradine hydrochloride tablet, and two cracked tablets fulfill the dosage of 2.5+ / -0.1mg after the tablet is torn according to the mark. The ivabradine hydrochloride tablet is prepared from ivabradine hydrochloride, lactose monohydrate, starch, PVPK30, aerosil and magnesium stearate. When a patient takes the tablet, the patient takes half tablet by tearing the tablet according to the mark so as to fulfill the dosage of 2.5mg, or takes the whole tablet to fulfill the dosage of 5mg, or takes one and a half tablets to fulfill the dosage of 7.5mg. Compared with the prior art, the ivabradine hydrochloride tablet and the preparation process have the advantages that a ivabradine hydrochloride tablet with small specification can meet the requirement of multiple usages and dosages, so that the patient compliance can be improved.
Owner:CISEN PHARMA
Novel ivabradine hydrochloride crystal form C and preparation method thereof
The invention belongs to the field of pharmaceutical chemicals, and relates to a novel ivabradine hydrochloride crystal form C and a preparation method of the novel ivabradine hydrochloride crystal form C. The preparation method comprises the following steps in sequence: heating ivabradine hydrochloride and ethanol to obtain a settled solution; adding ethyl acetate; stirring and devitrifying in a cooling condition; filtering; and collecting the product which is the ivabradine hydrochloride crystal form C. The novel ivabradine hydrochloride crystal form C provided by the invention is different from all publicized crystal forms in a crystal morphology, and has the advantages of being clear and reproducible in outline, relatively high in process stability, simple and convenient in operation, and relatively high in product stability.
Owner:江苏宇田医药有限公司
Pharmaceutical composition for treating chronic stable angina pectoris with hyperlipemia
ActiveCN101559228ALower levelReduce generationMetabolism disorderMacromolecular non-active ingredientsHMG-CoA reductaseIVABRADINE HYDROCHLORIDE
The invention relates to a pharmaceutical composition for treating chronic stable angina pectoris with hyperlipermia, which comprises the components of a drug carrier, a sino atrial node If current selective specificity inhibitor or pharmaceutical salt thereof, and an HMG-CoA reductase selective depressant or pharmaceutical salt thereof; the sino atrial node If current selective specificity inhibitor or the pharmaceutical salt thereof is Ivabradine hydrochloride; and the contents of the components of the medical composition by mass unit are 2.5 to 10 of the sino atrial node If current selective specificity inhibitor and 2.5 to 100 of the HMG-CoA reductase selective depressant. The medical composition has good synergistic effect when treating the chronic stable angina pectoris with the hyperlipermia, and has good effect of lowering hyperlipemia for hyperlipoidemia and hemorheology effect.
Owner:CSPC OUYI PHARM CO LTD
Ivabradine hydrochloride impurity and preparation method and application thereof
InactiveCN105669554AHigh purityEnsure safetyOrganic chemistryComponent separationIVABRADINE HYDROCHLORIDESide effect
The invention discloses an ivabradine hydrochloride impurity and a preparation method and application thereof. The impurity has thermal sensitivity, it is found in preparation stability tests that the impurity content is gradually increased along with inspection time increasing, specially when the storage temperature is higher than 25 DEG C, the impurity content is obviously increased, the quality of ivabradine hydrochloride is restricted, and potential toxic and side effects exist. The ivabradine hydrochloride impurity can be used for quality control over the ivabradine hydrochloride raw material and a preparation thereof, and the safety and effectiveness of using a product in clinic are guaranteed. The method for preparing the ivabradine hydrochloride impurity is easy to operate, the reaction condition is mild, and the high-purity ivabradine hydrochloride impurity can be obtained.
Owner:徐建立
Ivabradine hydrochloride form iv
ActiveUS20140315890A1Easy to handleImprove the preparation effectBiocideOrganic chemistry methodsIVABRADINE HYDROCHLORIDEBULK ACTIVE INGREDIENT
Ivabradine hydrochloride Form IV, its pharmaceutical composition, process for its preparation, and its use as therapeutically active ingredient.
Owner:URQUIMA
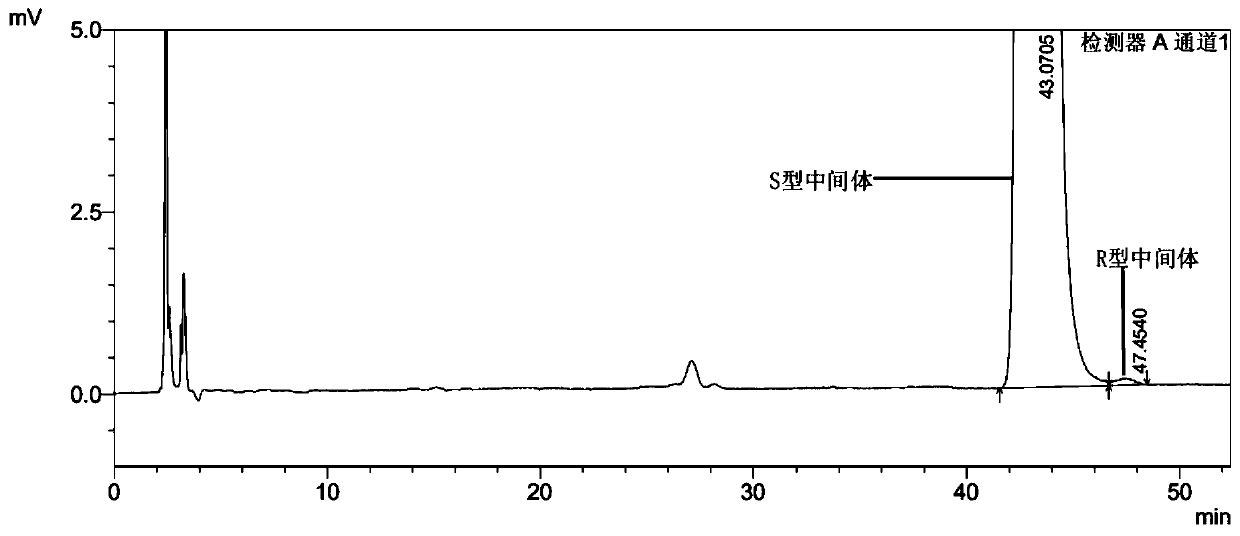
Detection method of enantiomer in ivabradine hydrochloride intermediate and application
ActiveCN111220727ASeparation detection is effectivePeak shape symmetryComponent separationEnantiomerColumn temperature
The invention provides a detection method of an enantiomer in an ivabradine hydrochloride intermediate, and an application. The detection method comprises the following steps of respectively preparingto-be-detected sample solutions, and injecting the to-be-detected sample solutions into a high performance liquid chromatograph under the detection conditions that a chromatographic column is a CHIRALPAK IC chromatographic column, the column temperature is 25-40 DEG C, the detection wavelength is 280-290 nm, a mobile phase is a mixture of normal hexane, dichloromethane and diethylamine in a volume ratio of (76-80):(20-24):0.5, and the flow rate of the mobile phase is 0.8-1.2 mL / min. According to the detection method disclosed by the invention, the enantiomer in the ivabradine hydrochloride intermediate can be precisely and quantitatively analyzed, the peak pattern is symmetrical and a tailing phenomenon does not exist, factors such as a separation degree, a quantification limit, a detection limit, repeatability, precision and solution stability all meet related requirements, the detection limit can reach 0.0337 mu g / mL, and enantiomer higher than 0.01% in the ivabradine hydrochlorideintermediate can be detected.
Owner:HAINAN XINOPEN SOURCE MEDICAL TECH CO LTD
New crystalline form of hydrochloric acid Ivabradine and preparation method thereof
The invention discloses a new crystalline form of hydrochloric acid Ivabradine and a preparation method thereof. A mixture of the hydrochloric acid Ivabradine, methanol and acetone is heated to be completely dissolved, natural stewing is carried out for complete crystallization, and products are collected through filtration so as to obtain the new crystalline form of the hydrochloric acid Ivabradine.
Owner:BEIJING D VENTUREPHARM TECH DEV
Novel ivabradine hydrochloride crystal form and its preparation method and use in preparation of pharmaceutical composition
InactiveCN102731400AHigh purityImprove stabilityOrganic chemistryHeterocyclic compound active ingredientsFiltrationAngina
The invention relates to a novel ivabradine hydrochloride crystal form and its preparation method and use in preparation of a pharmaceutical composition. The preparation method comprises the following steps of carrying out heating reflux stirring of a mixture of ivabradine hydrochloride and anhydrous ethanol, or a mixture of ivabradine hydrochloride, anhydrous ethanol and ethyl acetate until complete dissolution, stopping heating, carrying out natural cooling and stirring for crystallization, and after complete crystallization, carrying out pumping filtration collection of products. The novel ivabradine hydrochloride crystal form can treat or prevent various local myocardial ischemia clinical symptoms such as angina, myocardial infarction and accompanying rhythmic disorder, and also can treat or prevent symptoms related to rhythmic disorder and especially to supraventricular rhythmic disorder.
Owner:SHANDONG NEWTIME PHARMA
Polymorphic forms of ivabradine hydrochloride
InactiveUS9120755B2Reduce moisturePolycrystalline material growthFrom normal temperature solutionsIVABRADINE HYDROCHLORIDEMedicinal chemistry
Stable crystalline Form II and stable crystalline Form III of ivabradine hydrochloride and processes for their preparation are disclosed.
Owner:CADILA HEALTHCARE LTD
Stable ivabradine hydrochloride tablet and preparation method thereof
ActiveCN109875969AOvercoming the problem of crystal conversion of raw materialsImprove stabilityPill deliveryPharmaceutical non-active ingredientsLACTITOL MONOHYDRATELACTOSE MONOHYDRATE
The invention discloses a stable ivabradine hydrochloride tablet. The stable ivabradine hydrochloride tablet comprises ivabradine hydrochloride, lactose monohydrate, a disintegrating agent, a bindingagent, a glidant and a lubricant, wherein the lactose monohydrate and the disintegrating agent are prepared into dry granules, then the dry granules are mixed with the ivabradine hydrochloride, the binding agent, the glidant and the lubricant, and the ivabradine hydrochloride tablet is obtained through compression. In addition, the invention also discloses a preparation method. The ivabradine hydrochloride tablet not only solves the problems of crystal transition of a raw material medicine and standard exceeding of related substances, but also has good product stability.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA +1
Ivabradine compound, preparation method and pharmaceutical composition thereof
ActiveCN102304088BImprove stabilityOrganic chemistryHeterocyclic compound active ingredientsIVABRADINE HYDROCHLORIDECyclobutane
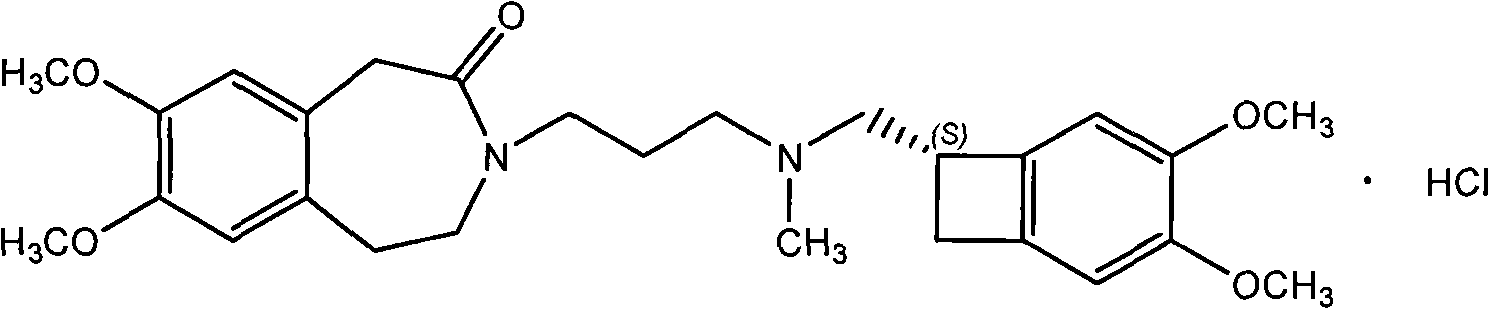
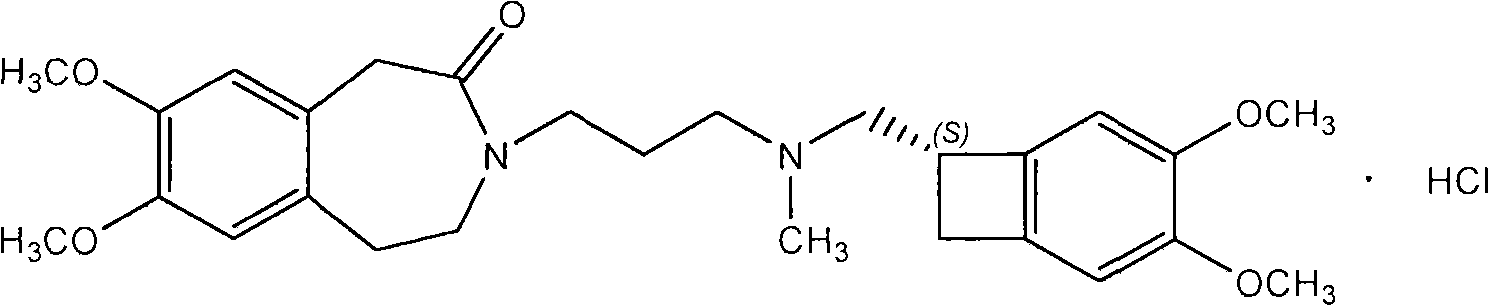
The invention relates to an ivabradine compound, a preparation method and a pharmaceutical composition thereof, in particular to 7,8-dimethoxy-3-{3-[[(1S)(4,5-dimethoxybenzo cyclobutane-1-yl)methyl]-methylamino]propyl}-1,3,4,5-tetrahydro-dihydro-benzoazepine-2-ketone, namely ivabradine hydrochloride hydrate, and further to a preparation method of the compound and a pharmaceutical composition by taking the compound as an active substance. Compared with the ivabradine hydrochloride, the compound disclosed by the invention has better stability and is better applied to preparing, storing and applying pharmaceutical preparations in various forms.
Owner:CSPC OUYI PHARM CO LTD
Ivabradine hydrochloride form IV
ActiveUS9139531B2Easy to handleImprove the preparation effectOrganic chemistry methodsCoatingsIVABRADINE HYDROCHLORIDEMedicine
Owner:URQUIMA
Preparation method of ivabradine hydrochloride crystal form variant DELTA-D
ActiveCN105503726AHigh purityGood storage stabilityOrganic chemistry methodsIVABRADINE HYDROCHLORIDEReaction temperature
The invention relates to a preparation method of an ivabradine hydrochloride crystal form variant DELTA-D, which comprises the following steps: 1) dissolving ivabradine hydrochloride in C2-C4 ketones; 2) heating the material obtained in the step 1) to 30-45 DEG C, and stirring to react for 6-50 hours; and 3) in a nitrogen atmosphere, filtering the reaction system obtained in the step 2), and drying the obtained solid at 40-85 DEG C to a dry state, wherein the water content in the reaction material in the step 1) is 0.1-1%. The control on the water content in the reaction material is used instead of the control on the relative humidity in the reaction process in the prior art, so that the method is convenient to control, does not need any special adjusting facility, and is convenient for implementing industrialization. The C2-C4 ketones are used as the solvent, thereby avoiding the toxic solvent residues in the crystal form. The reaction temperature is controlled at 25-45 DEG C, the reaction time is controlled at 6-50 hours, and the drying is performed to further enhance the purity of the crystal form. The DELTA-D crystal form prepared by the method has favorable storage stability.
Owner:ZHEJIANG MENOVO PHARMA
Amorphous ivabradine hydrochloride and preparation method thereof
InactiveCN104829530AHigh yieldImprove product qualityOrganic chemistryCardiovascular disorderIVABRADINE HYDROCHLORIDEMedicinal chemistry
The invention relates to the field of medicinal chemistry, and discloses a new amorphous ivabradine hydrochloride, a preparation method thereof and a pharmaceutical composition containing the same. The amorphous ivabradine hydrochloride has good stability. The preparation method for amorphous ivabradine hydrochloride provided by the invention is easy to operate, and has high amorphous ivabradine hydrochloride yield, thus being suitable for laboratory development and industrial production, and having wide application. The amorphous ivabradine hydrochloride product prepared by the method provided by the invention has stable quality and can be directly used for preparation of heart rate decreasing drugs.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Ivabradine hydrochloride osmotic pump controlled-release tablet and preparation method thereof
ActiveCN104398486AImprove stabilityMature technologyPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDrug releaseBULK ACTIVE INGREDIENT
The invention provides an ivabradine hydrochloride osmotic pump controlled-release tablet and a preparation method thereof. The osmotic pump controlled-release tablet is characterized by containing active ingredients of ivabradine hydrochloride and comprising a double-layer tablet core and a semipermeable coating membrane, wherein the double-layer tablet core is formed by pressing a high polymer material, osmotic pressure active substances and the like. The ivabradine hydrochloride osmotic pump controlled-release tablet enables the ivabradine hydrochloride to be slowly released at a constant speed in 0-24 hours, the release rate is more than 90%, and the osmotic pump controlled-release tablet has a long-acting zero-level drug-releasing characteristic. The osmotic pump controlled-release tablet can be used for treating patients suffering from chronic angina pectoris, stabilize the blood concentration, reduce adverse reactions caused by large fluctuation of the blood concentration and improve drug safety, and meanwhile reduces drug taking times of the patients and improves adaptability of the patients.
Owner:WUHAN WUYAO SCI & TECH
Preparation method and application of ivabradine structural analogue or acid salt thereof
PendingCN109970647AHigh puritySimple and fast operationOrganic chemistryIVABRADINE HYDROCHLORIDEChemical reaction
The invention discloses a preparation method of an ivabradine structural analogue or acid salt thereof. The preparation method comprises the step that a compound shown in formula (II) and an oxidizingagent are subjected to an oxidizing reaction in a solvent to obtain the ivabradine structural analogue shown in formula (I). The preparation method of the ivabradine structural analogue or acid saltthereof has the advantages that an impurity contrast can be provided for the quality control of ivabradine hydrochloride and security detection of clinical medication, and so that the safe and reliable clinical medication is ensured; the operation is simple and convenient, the reaction conditions are mild, the yield is relatively high, and the high-purity product can be obtained through a one-stepchemical reaction.
Owner:重庆德诚永道医药有限公司 +1
Ivabradine compound, preparation method and pharmaceutical composition thereof
ActiveCN102304088AStable characteristicsIncrease production capacityOrganic chemistryHeterocyclic compound active ingredientsIVABRADINE HYDROCHLORIDECyclobutane
The invention relates to an ivabradine compound, a preparation method and a pharmaceutical composition thereof, in particular to 7,8-dimethoxy-3-{3-[[(1S)(4,5-dimethoxybenzo cyclobutane-1-yl)methyl]-methylamino]propyl}-1,3,4,5-tetrahydro-dihydro-benzoazepine-2-ketone, namely ivabradine hydrochloride hydrate, and further to a preparation method of the compound and a pharmaceutical composition by taking the compound as an active substance. Compared with the ivabradine hydrochloride, the compound disclosed by the invention has better stability and is better applied to preparing, storing and applying pharmaceutical preparations in various forms.
Owner:CSPC OUYI PHARM CO LTD
A kind of ivabradine hydrochloride osmotic pump controlled-release tablet and preparation method thereof
ActiveCN104398486BSmall toxicityControl releasePharmaceutical delivery mechanismPharmaceutical non-active ingredientsPatient complianceBULK ACTIVE INGREDIENT
The invention provides an ivabradine hydrochloride osmotic pump controlled-release tablet and a preparation method thereof. The osmotic pump controlled-release tablet of the invention is characterized in that it contains the active ingredient of ivabradine hydrochloride, and is composed of a double-layer tablet core compressed by a polymer material, an osmotic pressure active substance and the like, and a semi-permeable coating film. The preparation enables ivabradine hydrochloride to achieve a constant-speed slow release within 0-24 hours, and the release rate exceeds 90%, and has the characteristics of long-acting zero-order drug release. The invention is used for treating patients with chronic angina pectoris, can stabilize blood drug concentration, reduce adverse reactions caused by large fluctuation of blood drug concentration, and improve drug safety; meanwhile, it reduces the number of patients taking the drug and improves the patient's compliance.
Owner:WUHAN WUYAO SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com