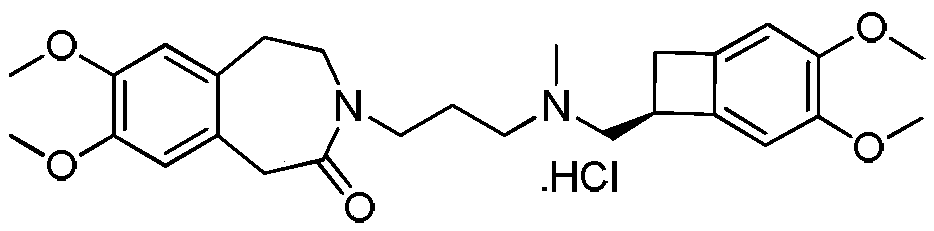
Preparation method of ivabradine hydrochloride crystal form variant DELTA-D
A technology of hydrochloride and crystal form, which is applied in the field of preparation of ivabradine hydrochloride crystal form variant DELTA-D, can solve the problems of product mixed crystals, difficult industrialization, and low product purity, and achieve increased purity, Ease of control and good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
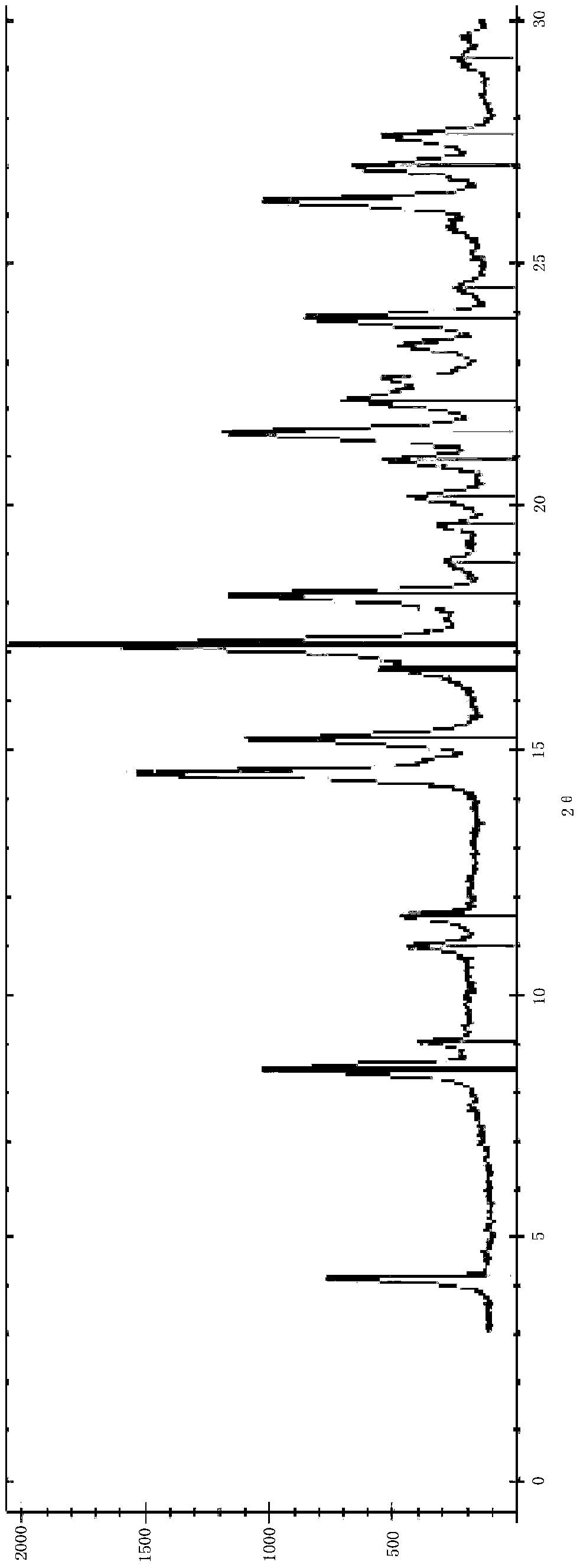
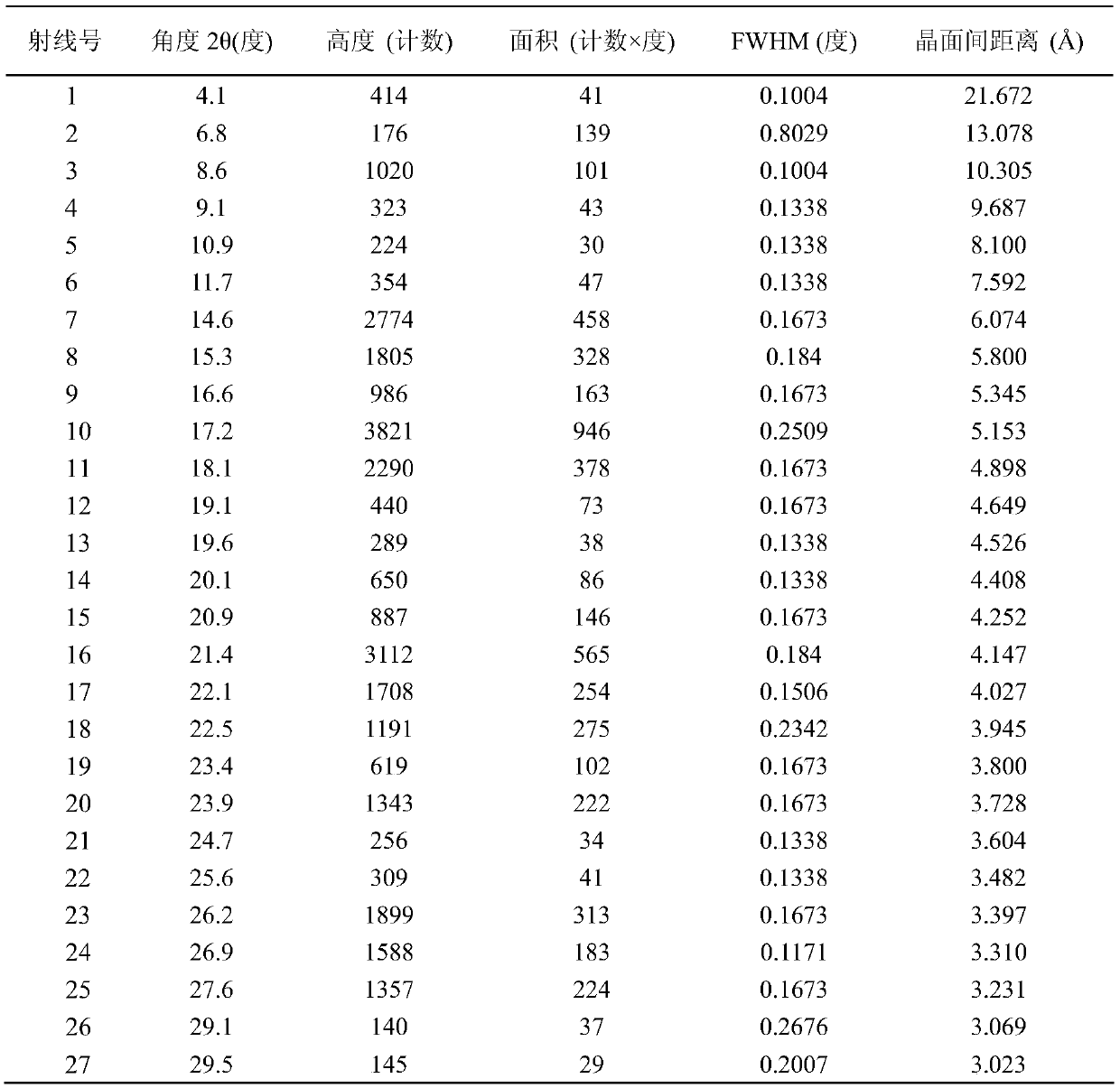
[0021] The present invention provides a method for preparing ivabradine hydrochloride crystal form variant DELTA-D, the XRD spectrum of the prepared DELTA-D crystal form is as follows figure 1 As shown, the 2θ peaks of the X-ray powder diffraction test are: 4.1, 8.6, 9.1, 10.9, 11.7, 14.6, 15.3, 16.6, 17.2, 18.1, 19.1, 19.6, 20.1, 20.9, 21.4, 22.1, 22.5, 23.4, 23.9 , 24.7, 25.6, 26.2, 26.9, 27.6, 29.1, 29.5. The specific data are shown in Table 1.
[0022] Table 1: X-ray powder diffraction spectrum characteristic peaks at 2θ of δ-d crystal form
[0023]
Embodiment 1
[0025] The preparation method of ivabradine hydrochloride crystal form modification DELTA-D in this embodiment comprises the following steps:
[0026] 1) Dissolving ivabradine hydrochloride crystal form α in acetone, wherein the water content of ivabradine hydrochloride crystal form α is 0.1%, the water content of acetone is 0.1%, and the amount of acetone is Ivabradine hydrochloride 5 times the dosage of bradine hydrochloride crystal form α;
[0027] 2) The temperature of the material obtained in step 1) was raised to 35°C, and stirred for 10 hours;
[0028] 3) Under a nitrogen atmosphere, filter the reaction system obtained in step 2), and dry the obtained solid under a vacuum environment, 0.8 MPa, 40°C, and dry for 24 hours.
[0029] After testing, the crystal form prepared in this example is pure crystal DELTA-D.
Embodiment 2
[0031] The preparation method of ivabradine hydrochloride crystal form modification DELTA-D in this embodiment comprises the following steps:
[0032] 1) Dissolving ivabradine hydrochloride crystal form δ in methyl ethyl ketone, wherein the water content of ivabradine hydrochloride crystal form δ is 0, and the water content of methyl ethyl ketone is 0.3 %, the amount of methyl ethyl ketone is 10 times that of ivabradine hydrochloride;
[0033] 2) The temperature of the material obtained in step 1) was raised to 30° C., and the reaction was stirred for 6 hours;
[0034] 3) Under a nitrogen atmosphere, filter the reaction system obtained in step 2), and dry the obtained solid under a vacuum environment, 1.0 MPa, 85° C., and dry for 12 hours.
[0035] After testing, the crystal form prepared in this example is pure crystal DELTA-D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com