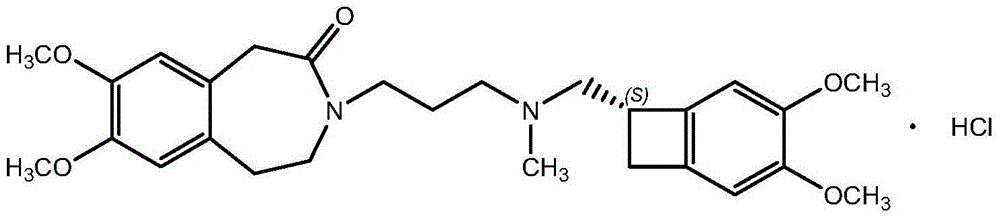
Amorphous ivabradine hydrochloride and preparation method thereof
An ivabradine hydrochloride, amorphous technology, applied in cardiovascular system diseases, active ingredients of heterocyclic compounds, drug combinations, etc., can solve problems such as easy water absorption, low production efficiency, instability, etc., and achieve simple operation and good performance , the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
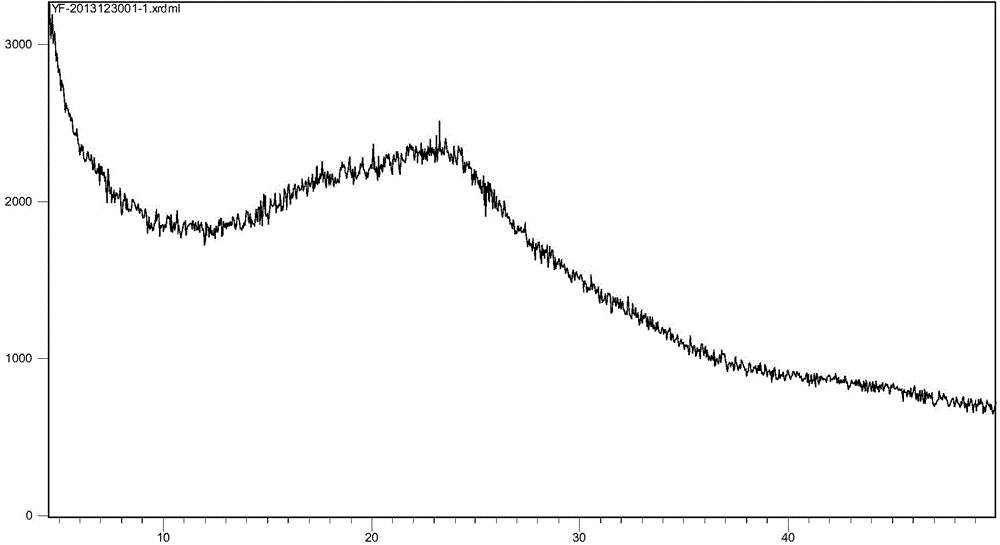
[0063] Mix 90ml of purified water and 10ml of absolute ethanol, then add 10g of ivabradine hydrochloride in batches, while stirring, until the solution is complete. The resulting clear solution was heated at 50 °C for an additional 0.5 h, then gradually cooled to ambient temperature. Turn on the freeze dryer and set the freeze-drying curve: -50°C, 10 5 Pa(4h); -20°C, 10Pa(12h); 0°C, 10Pa(2h); 15°C, 10Pa(2h); 30°C, 10Pa(2h); 50°C, 10Pa(10h), the product was 9.7g , which was detected as an amorphous powder by XRD, and the test results were as follows figure 1 .
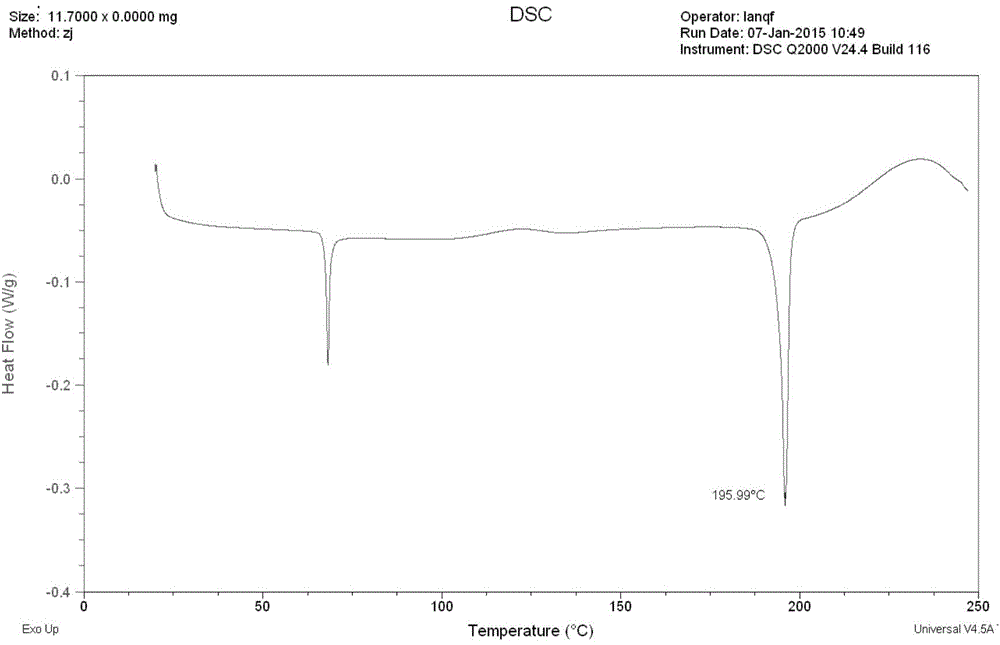
[0064] The amorphous ivabradine hydrochloride prepared in Example 1 was characterized by differential scanning calorimetry (DSC). see results figure 2 .
[0065] Depend on figure 2 The results show that the differential scanning calorimetry curve has an endothermic peak at about 195.99°C.
Embodiment 2
[0067] Mix 70ml of purified water and 30ml of absolute ethanol, then add 10g of ivabradine hydrochloride in batches, while stirring, until the solution is complete. The resulting clear solution was heated at 50 °C for an additional 0.5 h, then gradually cooled to ambient temperature. Turn on the freeze dryer, set the freeze-drying curve -40°C, 10 5 Pa(4h); -30℃, 10Pa(12h); 0℃, 10Pa(2h); 10℃, 10Pa(2h); 30℃, 10Pa(2h); 50℃, 10Pa(10h), the product was 9.6g , which was detected as an amorphous powder by XRD, and the test results were as follows figure 1 , DSC detection result is the same as embodiment 1.
Embodiment 3
[0069] Mix 90ml of purified water and 10ml of tert-butanol, then add 10g of ivabradine hydrochloride in batches, while stirring, until the solution is complete. The resulting clear solution was heated at 50 °C for an additional 0.5 h, then gradually cooled to ambient temperature. Turn on the freeze dryer and set the freeze-drying curve: -60°C, 10 5 Pa(4h); -30℃, 10Pa(12h); 0℃, 10Pa(2h); 20℃, 10Pa(2h); 40℃, 10Pa(2h); 80℃, 10Pa(10h), the product 9.6g was obtained , which was detected as an amorphous powder by XRD, and the test results were as follows figure 1 , DSC detection result is the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com