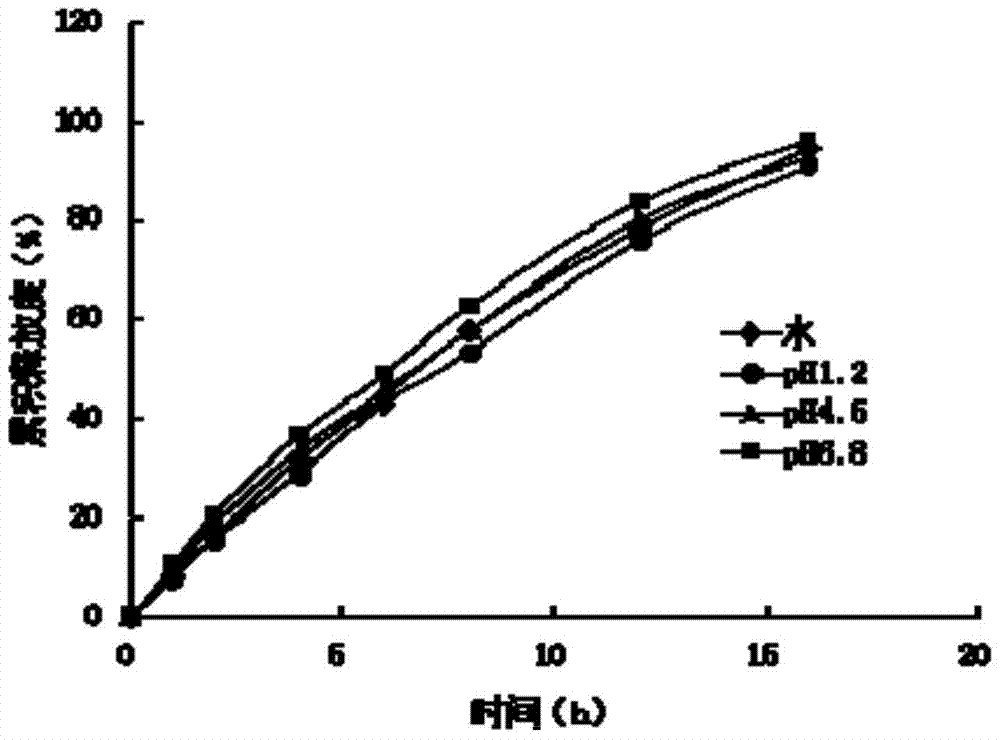
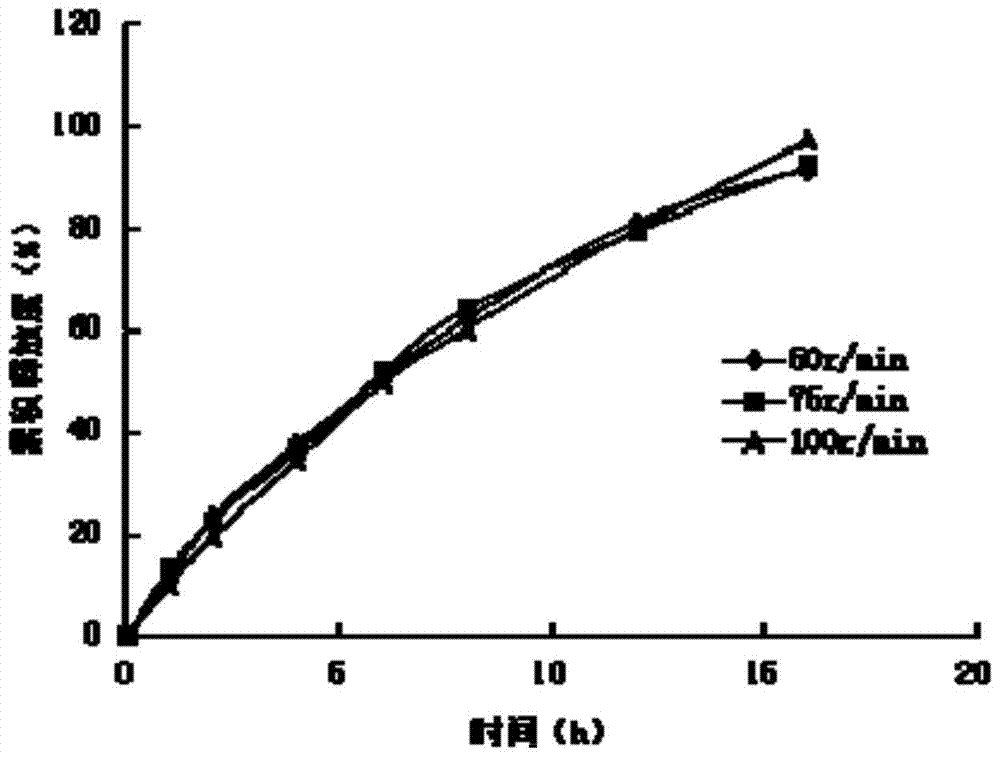
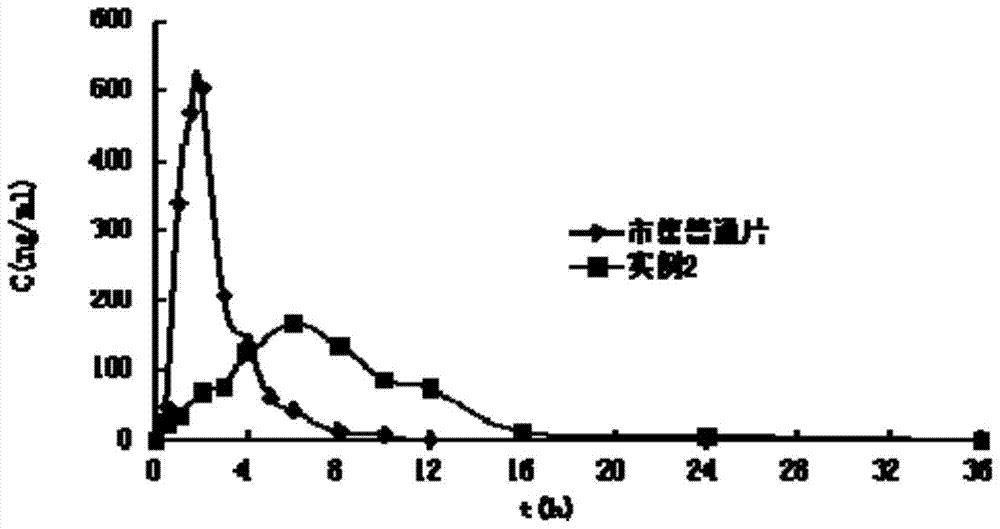
A kind of ivabradine hydrochloride osmotic pump controlled-release tablet and preparation method thereof
A technology of ivabradine hydrochloride and osmotic pump controlled release, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, cardiovascular system diseases, etc. The effect of reducing the number of doses, maturing the process, improving compliance and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Tablet prescription:
[0032]
[0033] Coating prescription
[0034] composition
Dosage / g
Cellulose acetate
100
polyethylene glycol 4000
10
Appropriate amount
water
Appropriate amount
[0035] Preparation Process:
[0036] Pass the drug and polyoxyethylene in the prescription amount of the drug-containing layer through a 80-mesh sieve, add a lubricant and mix evenly; pass the prescription amount of polyoxyethylene, sodium chloride and magnesium stearate in the booster layer through a 80-mesh sieve, and add a lubricant Mix evenly, use the No. 8 punch twice to pressurize to get the tablet core. Cellulose acetate and polyethylene glycol were dissolved in acetone and distilled water respectively and mixed evenly, and the tablet core was coated with a coating pan with a weight gain of 6%. After coating, it was cured in an oven at 40°C for 12 hours. Then prepare and punch a 0.6mm diameter release hole o...
Embodiment 2
[0038] Tablet prescription:
[0039]
[0040] Coating film prescription:
[0041] composition
Dosage / g
100
polyethylene glycol 4000
12.5
Appropriate amount
Appropriate amount
[0042] Preparation Process:
[0043] Pass the drug, polyoxyethylene, and lactose in the prescription amount of the drug-containing layer through a 80-mesh sieve, add lubricant, and mix evenly; pass the prescription amount of polyoxyethylene and sodium chloride in the booster layer through an 80-mesh sieve, add lubricant and mix evenly , Use the No. 8 punch to pressurize twice to get the tablet core. Dissolve ethyl cellulose and polyethylene glycol in acetone and distilled water respectively and mix evenly, coat the tablet core with a coating pan, with a weight gain of 6%, and cure in an oven at 40°C for 12 hours after coating . Then prepare a 0.6mm diameter drug release hole on the drug-containing ...
Embodiment 3
[0045]Tablet prescription:
[0046]
[0047] Coating film prescription:
[0048] composition
Dosage / g
Cellulose acetate
100
polyethylene glycol 4000
12.5
acetone
Appropriate amount
water
Appropriate amount
[0049] Preparation Process:
[0050] Pass the drug, polyoxyethylene, and mannitol in the prescription amount of the drug-containing layer through a 80-mesh sieve, add lubricant and mix evenly, pass the prescription amount of polyoxyethylene and sodium chloride in the booster layer through an 80-mesh sieve, add lubricant and mix evenly , Use the No. 8 punch to pressurize twice to get the tablet core. Cellulose acetate and polyethylene glycol were dissolved in acetone and distilled water respectively and mixed evenly, and the tablet core was coated with a coating pan, with a weight gain of 8%. After coating, it was cured in an oven at 40°C for 12 hours. Then prepare a 0.6mm diameter release hole on the side of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com