Detection method of enantiomer in ivabradine hydrochloride intermediate and application
A technology of ivabradine hydrochloride and enantiomers, which is applied in the field of detection of enantiomers in ivabradine hydrochloride intermediates, to achieve good detection effect, symmetrical peak shape and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
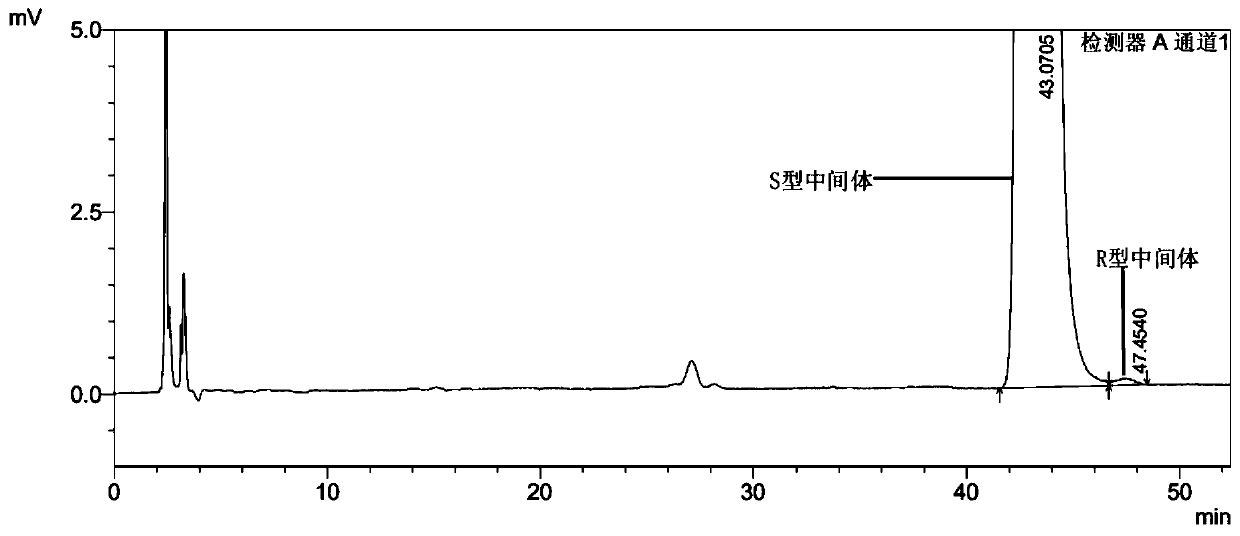
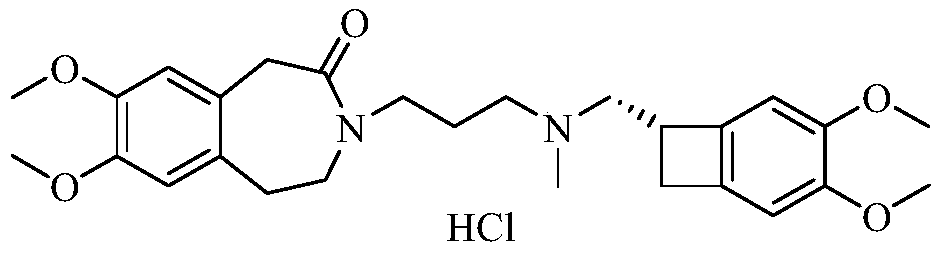
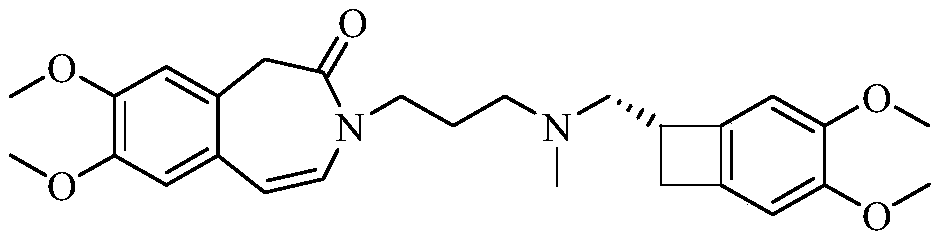
[0045] Embodiment 1-In order to carry out quality control to the key intermediate of ivabradine hydrochloride, and continuously improve the safety and effectiveness of ivabradine hydrochloride medicines, the present invention proposes a kind of ivabradine hydrochloride intermediate enantiomer The detection method of isomers, the intermediate of ivabradine hydrochloride is 3-[3-[[[(7S)-3,4-dimethoxybicyclo[4.2.0]octane-1,3,5-tri En-7-yl]methyl]methylamino]propyl]-1,3-dihydro-2H-benzazepin-2-one, whose enantiomer is 3-[3-[[[ (7R)-3,4-Dimethoxybicyclo[4.2.0]oct-1,3,5-trien-7-yl]methyl]methylamino]propyl]-1,3-dihydro -2H-benzazepin-2-one, its detection method is:
[0046] Prepare the sample solution to be tested respectively: the reference substance solution of the enantiomer, the test solution of the ivabradine hydrochloride intermediate and the mixed solution of the ivabradine hydrochloride intermediate and the enantiomer, inject the high-efficiency chromatographic The determi...
Embodiment 2
[0047] The detection method of enantiomer in embodiment 2-a kind of ivabradine hydrochloride intermediate, comprises the steps:
[0048] (1) Prepare the sample solution to be tested:
[0049] S1. Preparation of 3-[3-[[[(7S)-3,4-dimethoxybicyclo[4.2.0]oct-1,3,5-trien-7-yl]methyl]methylamino ]Propyl]-1,3-dihydro-2H-benzazepin-2-one for the test solution:
[0050] Take ivabradine hydrochloride intermediate sample and mobile phase and mix and be mixed with need testing solution, stand-by, according to mass volume ratio (g / L), ivabradine hydrochloride intermediate sample: the ratio of mobile phase is ( 0.1~5):1.
[0051] S2. Preparation of 3-[3-[[[(7R)-3,4-dimethoxybicyclo[4.2.0]oct-1,3,5-trien-7-yl]methyl]methylamino ]Propyl]-1,3-dihydro-2H-benzazepin-2-one reference substance stock solution:
[0052] Take by weighing enantiomers and add in volumetric flask, mix with mobile phase and prepare reference substance stock solution, stand-by, according to mass volume ratio (g / L), en...
Embodiment 3
[0070] Select 3-[3-[[[(7S)-3,4-dimethoxybicyclo[4.2.0]oct-1,3,5-trien-7-yl]methyl] with batch number 191104 Methylamino] propyl] -1,3-dihydro-2H-benzazepin-2-one content is detected, comprising the following steps:
[0071] S1, prepare the test solution of ivabradine hydrochloride intermediate:
[0072] Take by weighing 10mg ivabradine hydrochloride intermediate sample and add 10mL mobile phase, after mixing uniformly, be mixed with the solution that every 1mL contains ivabradine hydrochloride intermediate 1.0mg, as need testing solution, stand-by.
[0073] S2, prepare the reference substance stock solution of enantiomer:
[0074] Weigh an appropriate amount of the reference substance of the enantiomer, weigh it precisely, dissolve it in the mobile phase and quantitatively dilute it to make a solution containing about 10 μg per 1 mL, as the stock solution of the reference substance of the enantiomer, and stand-by.
[0075] S3, prepare the reference substance solution of enan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com