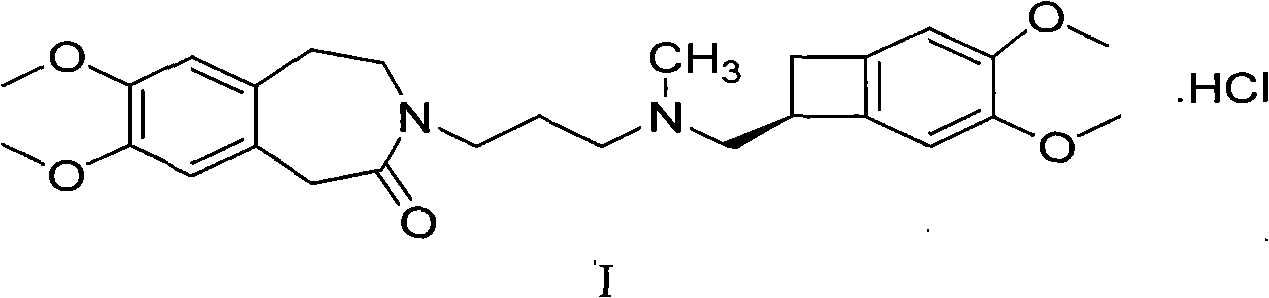
Omega-crystal form, preparation method and medicine composite of ivabradine hydrochloride
A technology of hydrochloride and crystal form, applied in the field of ω-crystal form of ivabradine hydrochloride, its preparation and pharmaceutical composition, capable of solving problems not specifically described in detail
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the omega-crystal form of ivabradine hydrochloride
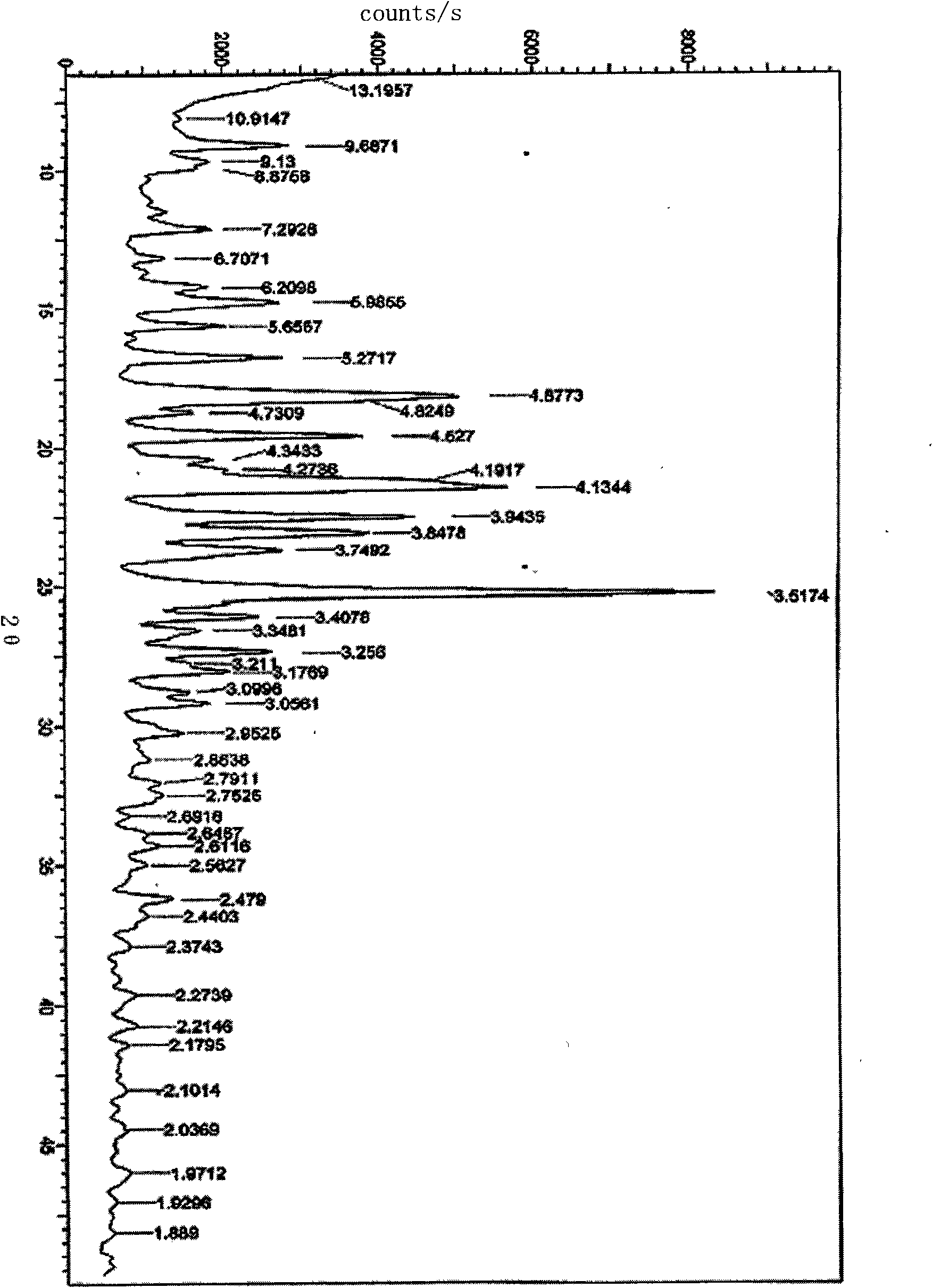
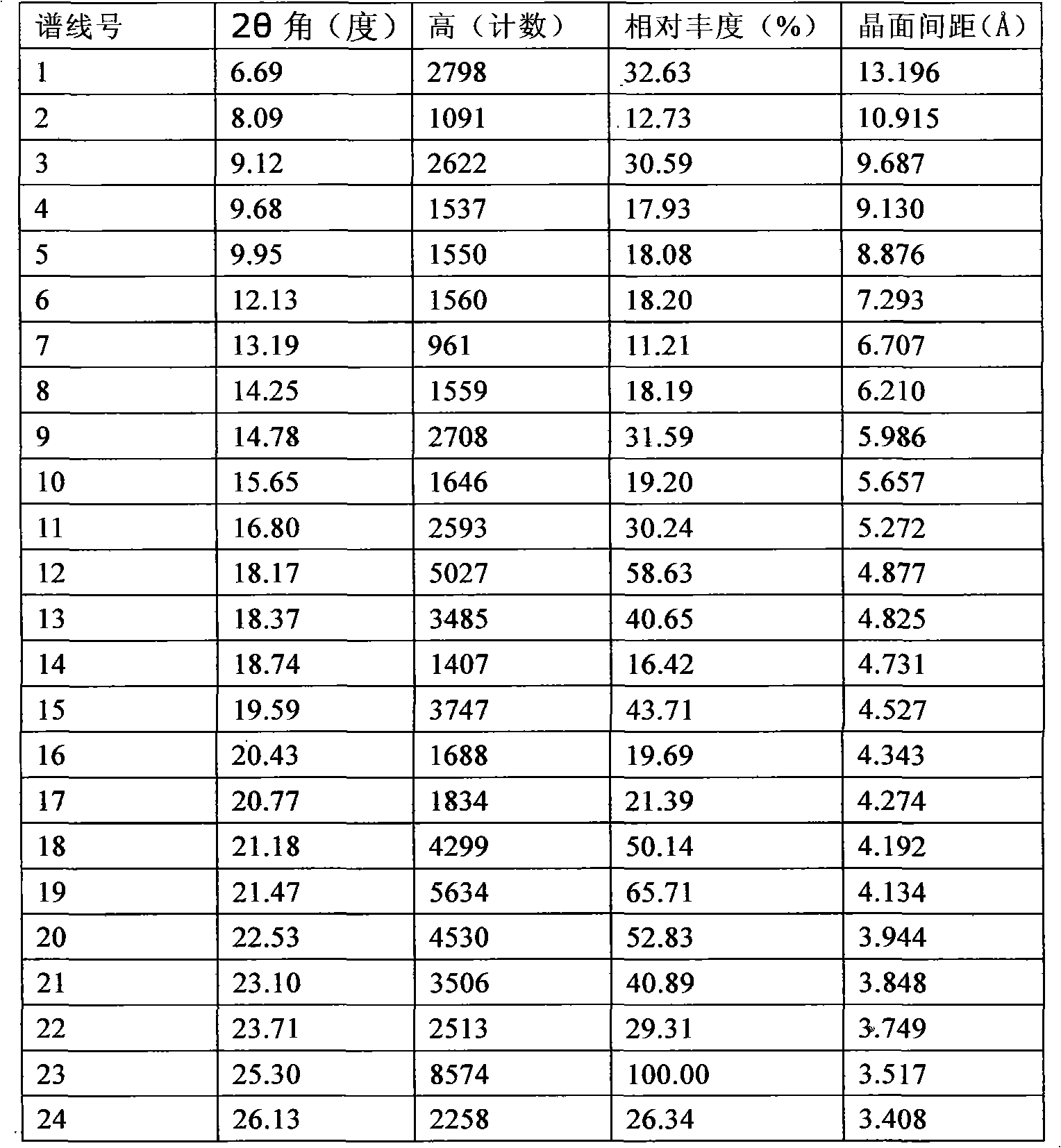
[0020] 20 grams of ivabradine hydrochloride obtained according to the method described in European patent specification EP0534859 was added to 30 ml of purified water, heated while stirring until completely dissolved. It is then cooled and freeze-dried. According to XRD it was shown that the ω-crystal form was generated.
Embodiment 2
[0021] Embodiment 2: pharmaceutical composition
[0022] Recipe for the preparation of 1000 tablets each containing 7.5 mg of febuxostat:
[0023] Compound 8.085g of Example 1
[0024] Cornstarch 30g
[0025] Mannitol 85g
[0026] PVP 11.5g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com