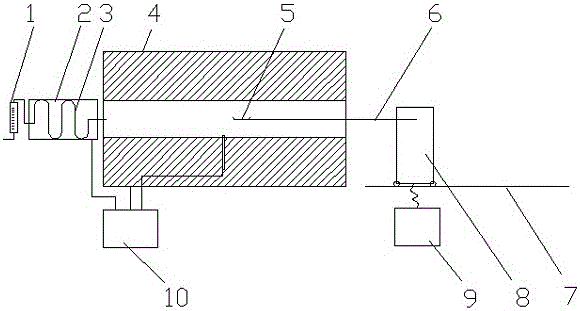
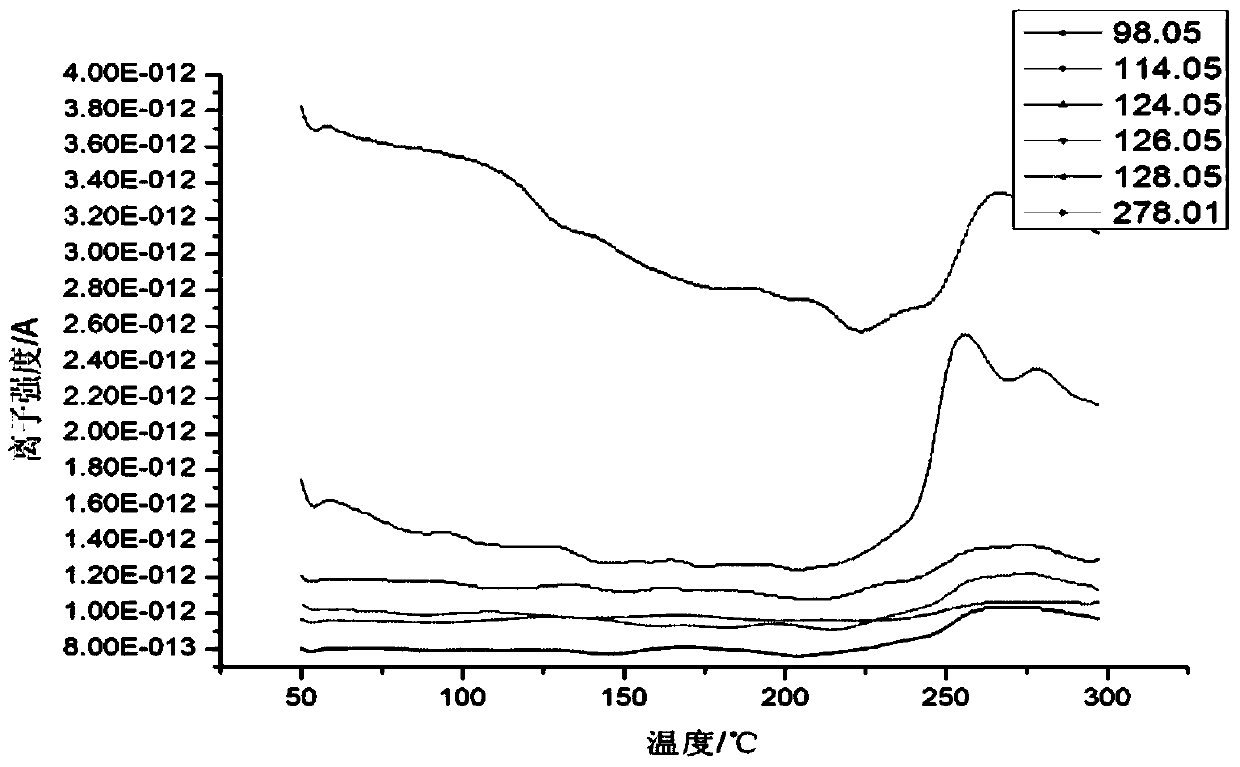
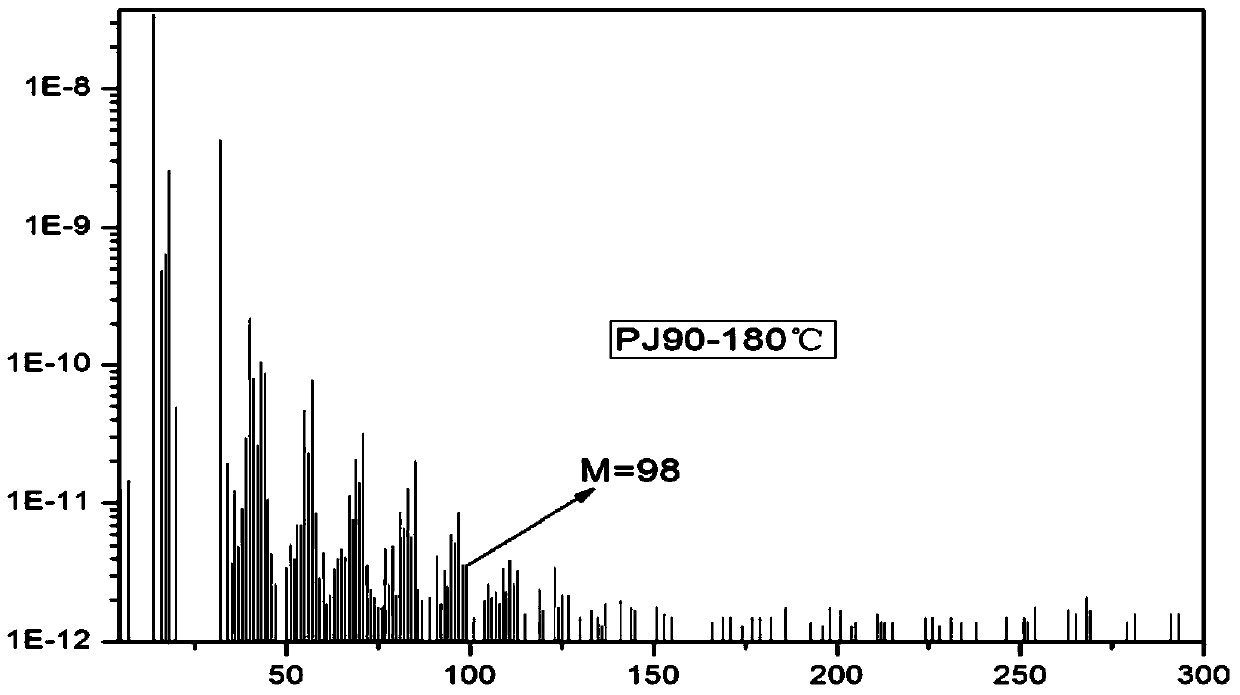
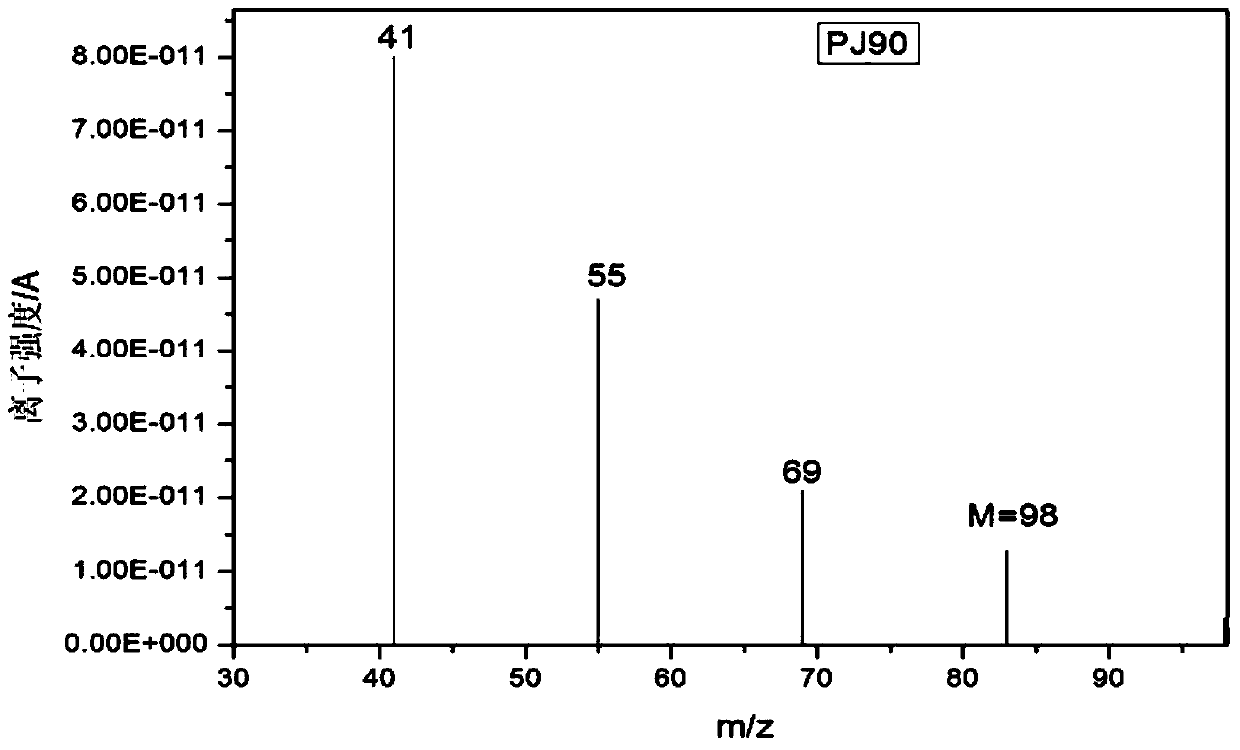
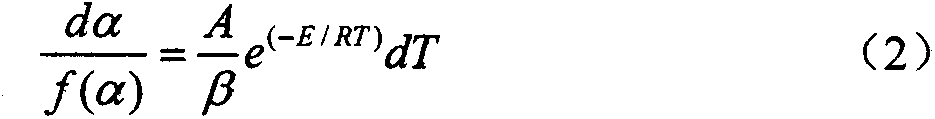Patents
Literature
151 results about "Thermogravimetry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thermogravimetry is a branch of physical chemistry, materials research, and thermal analysis. It is based on continuous recording of mass changes of a sample of material, as a function of a combination of temperature with time, and additionally of pressure and gas composition.
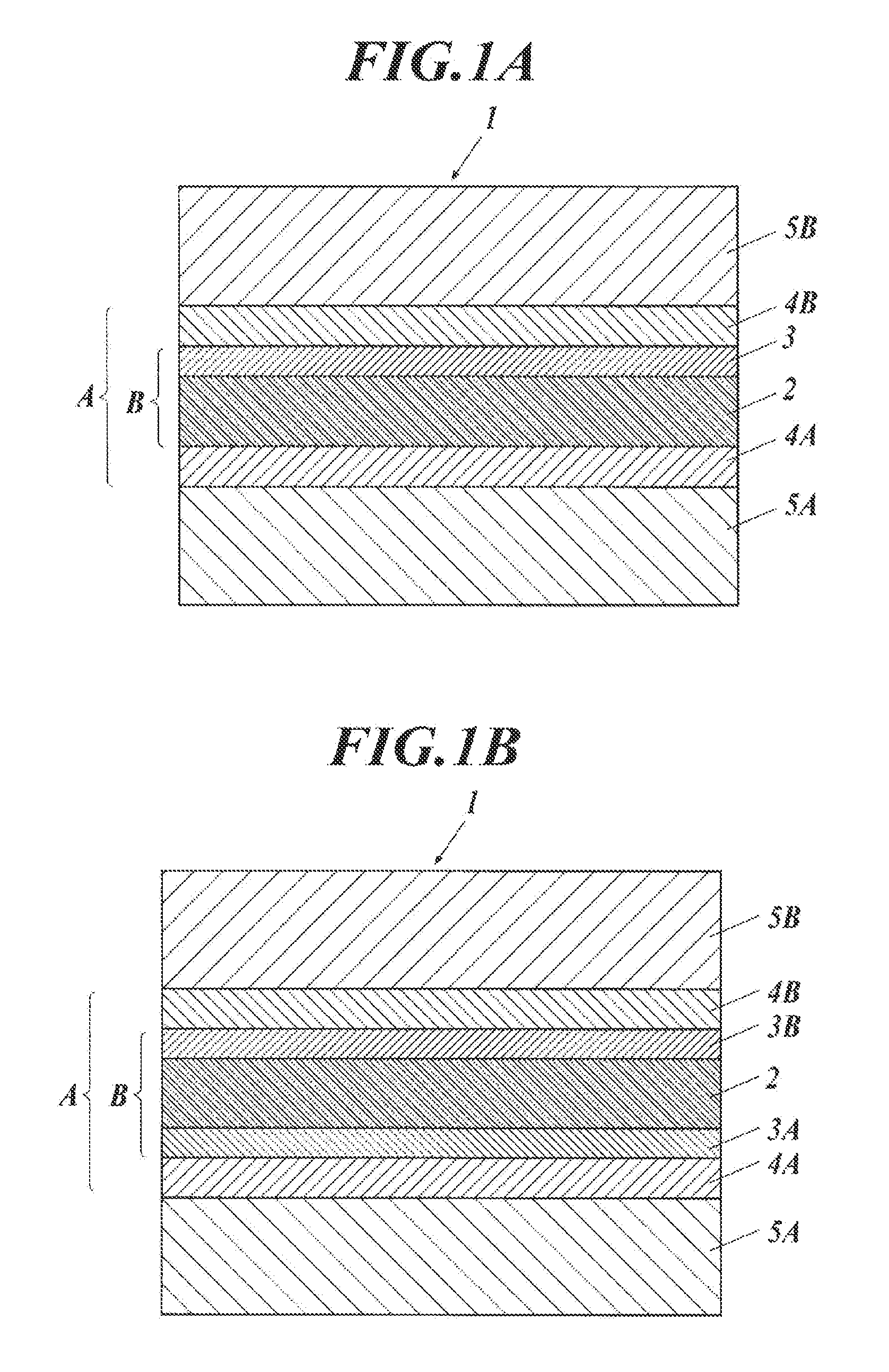
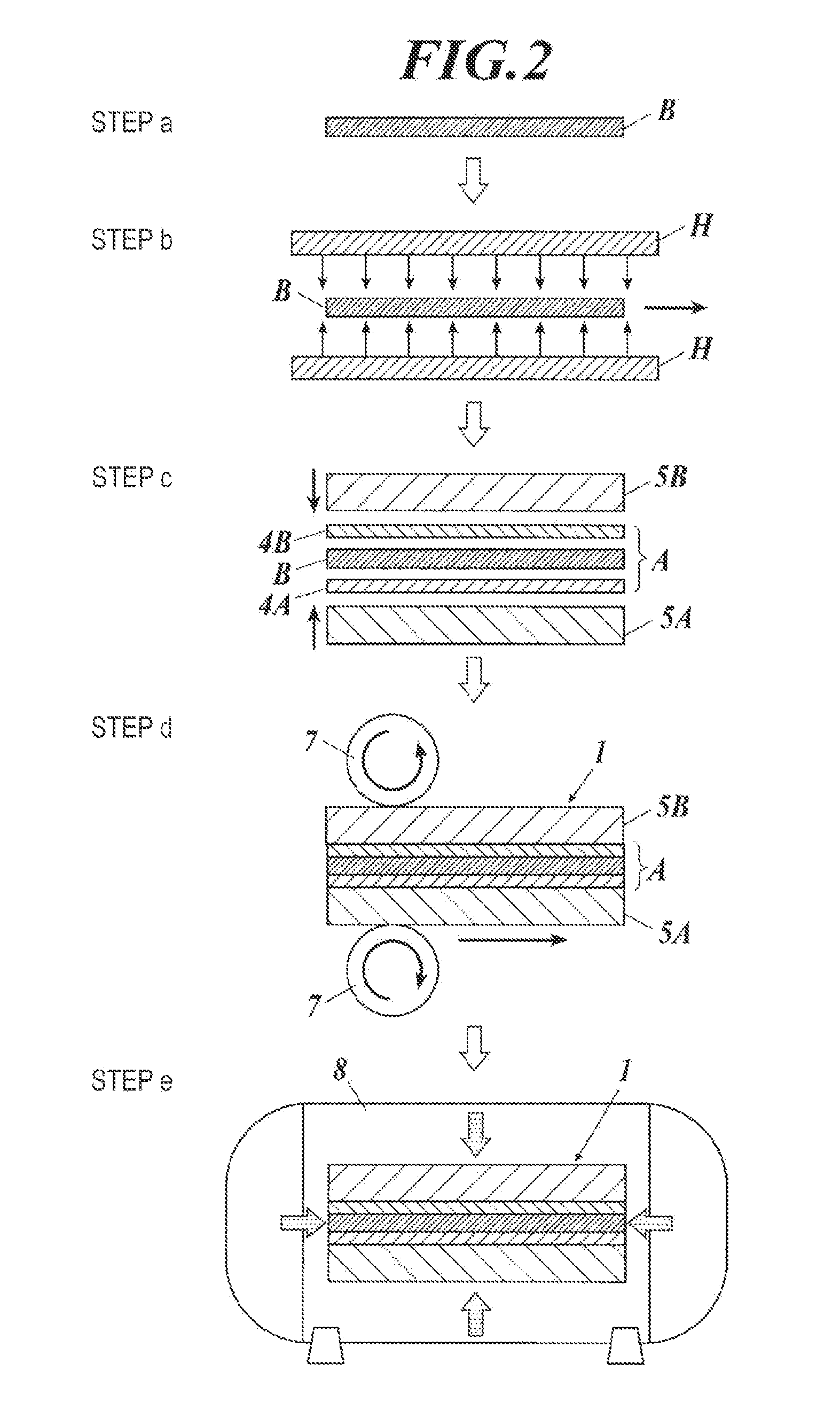
Carbon nanotube assembly and process for producing the same
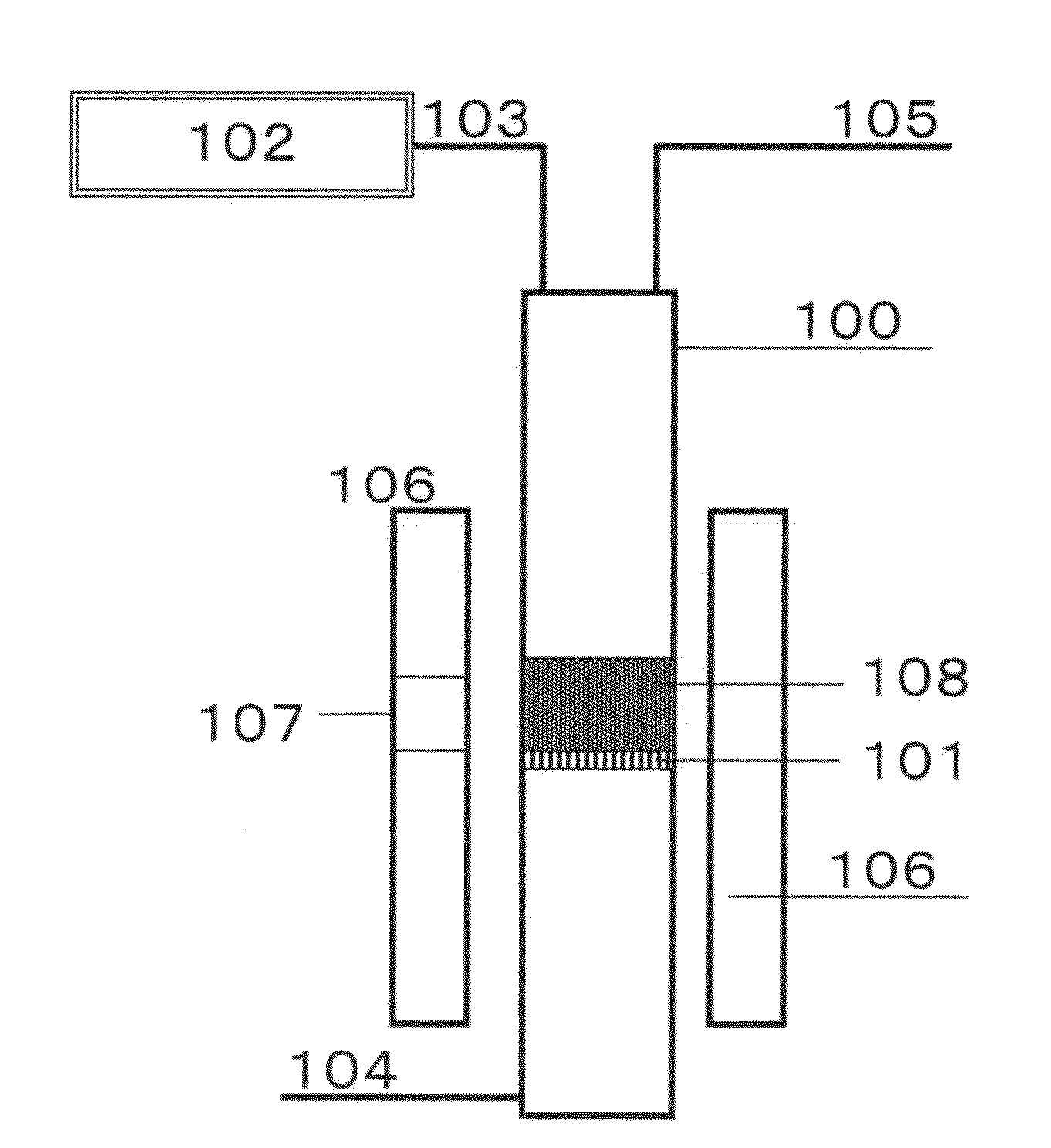
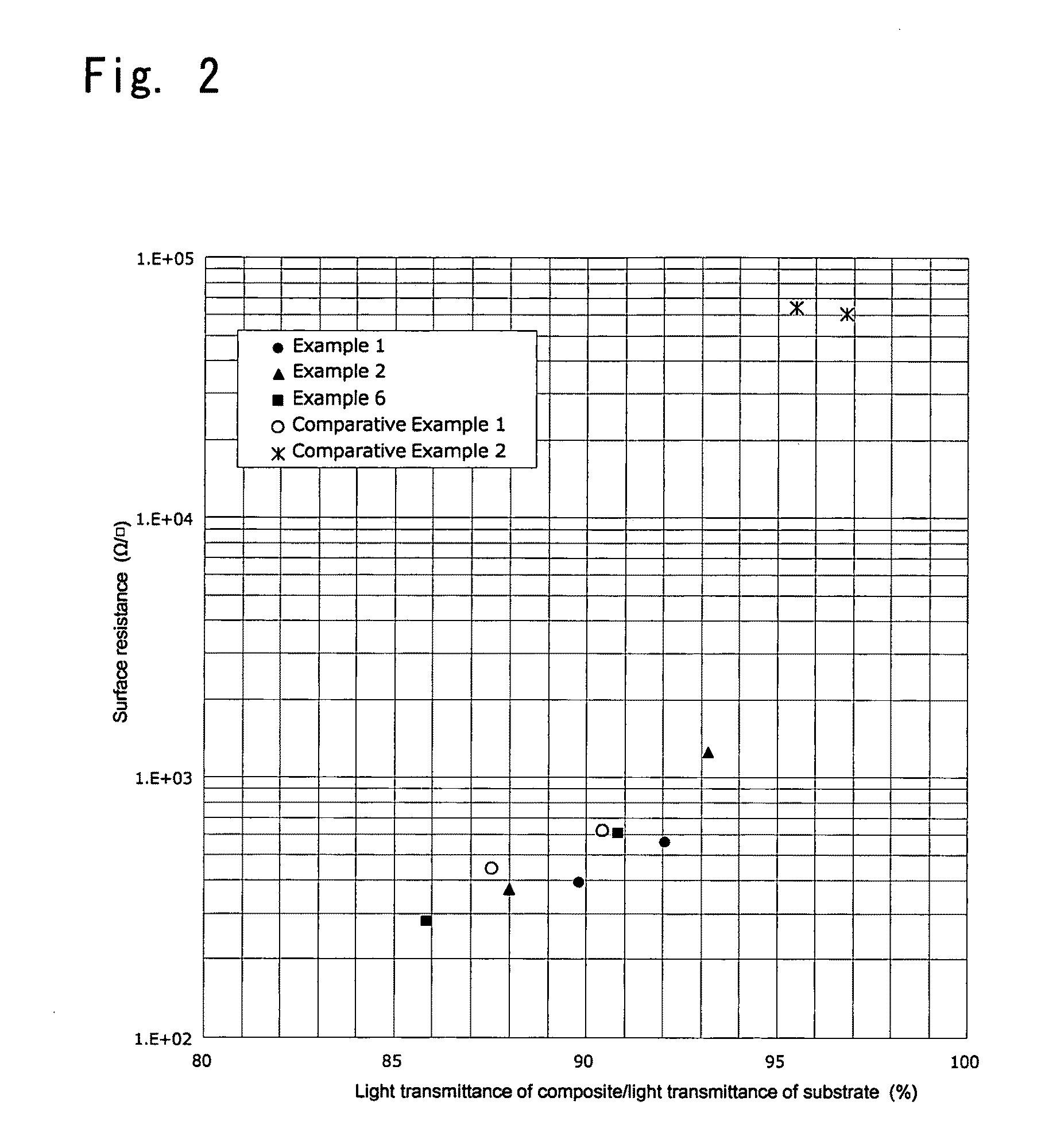
ActiveUS20100301278A1High light transmittanceImprove surface resistanceMaterial nanotechnologyLayered productsElectron microscopeMaterials science
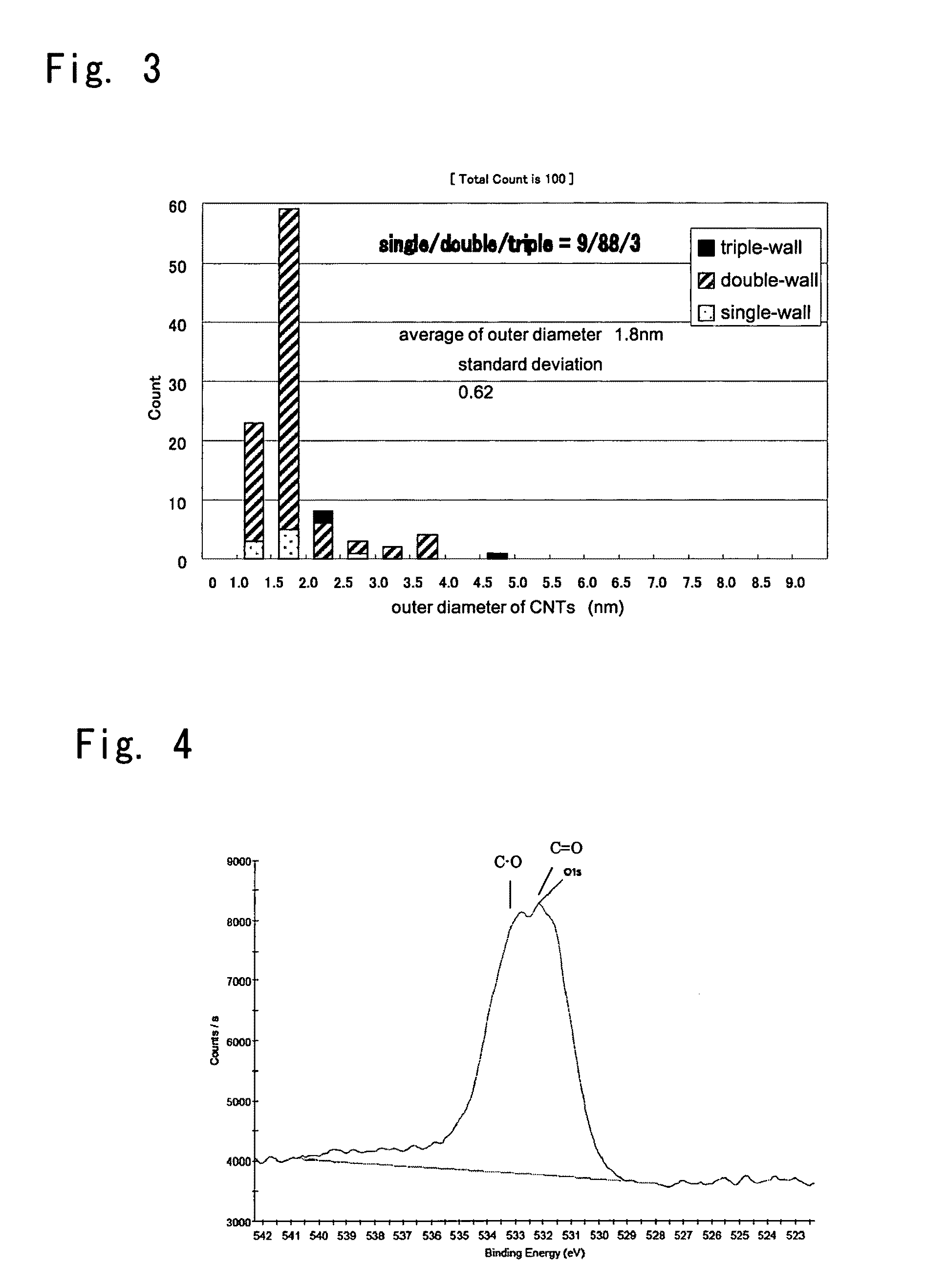
An aggregate of carbon nanotubes satisfying all of the following requirements (1) to (3):(1) the volume resistivity is from 1×10−5 Ω·cm to 5×10−3 Ω·cm;(2) at least 50 out of 100 carbon nanotubes are double-walled carbon nanotubes in observation by a transmission electron microscope; and(3) the weight loss from 200° C. to 400° C. in thermogravimetry at a temperature rise of 10° C. / min is from 5% to 20%.
Owner:TORAY IND INC
Surface carboxyl functionalized polystyrene / nano silicon dioxide hybridization material and preparation thereof
The invention discloses a method for preparing surface carboxylic group functional polystyrene / nano-silicon dioxide hybrid material by effectively combining click chemistry and ATRP; in the method, a silane coupling agent is adopted for linking an ATRP evocating agent to a SiO2 particle surface, and polystyrene (PSt) is grafted on the SiO2 particle surface by adopting the ATRP; then, carboxyl is led into a nano-silicon dioxide particle surface modified by the polystyrene by means of the click reaction, thus obtaining the surface carboxylic group functional polystyrene / nano-silicon dioxide hybrid material. After the infrared, nuclear magnetism and thermogravimetry mapping analysis, the surface carboxylic group functional polystyrene / nano-silicon dioxide hybrid material prepared by the invention has good thermal stability and dispersivity; in addition, as the carboxyl has wide reaction range and the characteristic of easy ionization, nano-particles has high reactive behavior so as to be widely applied to the fields such as high polymer material modifying agents, water treatment agents, catalysts, sensing agents and protein carriers.
Owner:NORTHWEST NORMAL UNIVERSITY
Carbon nanotube assembly and process for producing the same
InactiveUS8038908B2Increase resistanceImprove transmittanceMaterial nanotechnologyLayered productsElectron microscopeMaterials science
Owner:TORAY IND INC
Application and method of TGA-IR-GCMS triple machine in tobacco analysis
The invention relates to an application and a method of a TGA-IR-GCMS triple machine (thermogravimetric analyzer-infrared spectrometer-gas chromatography mass spectrum) in tobacco analysis. A thermogravimetric-infrared-gas chromatography mass spectrum (STA-IR-GC-MS) triple machine is employed to perform thermogravimetry-infrared-gas chromatography mass spectrum analysis for components of cigarette smoke. A cigarette sample is placed in a thermogravimetric sample cup; high-purity nitrogen or an oxygen-nitrogen gas mixture is introduced into the thermogravimetric analyzer; preliminary determination of the structure is performed by purging pyrolysis products of the sample in such atmosphere by the gas into an online-detecting infrared spectrometer; then accurate qualitative determination of the cigarette sample is performed by purging the products by the gas into a GC-MS for detection; and pyrolysis products of different time points and temperature points are analyzed. The method of the invention has the characteristics of simple operation, rapid analysis speed, comprehensive detection and the like, can perform online detection, analysis and qualitative determination of the pyrolysis products of the cigarette, and provides a scientific and reliable analysis method for mechanism and principle research in aspects of cigarette additives, safety evaluation, harm reduction and tar reduction, etc.
Owner:YUNNAN RES INST OF TOBACCO SCI
Process for producing polyether polyol
InactiveUS20080071118A1Less coloringOrganic compound preparationEther preparation from oxiranesPolyolDesorption
To provide a method of producing a polyether polyol having less coloration with good selectivity and high efficiency by dehydrocondensing a polyol. In producing a polyether polyol by dehydrocondensation reaction of a polyol, a solid acid catalyst satisfying at least one of the following requirements (1) to (3) is used: (1) Acid function H0 measured by Hammett's indicator adsorption method is larger than −3; (2) In Temperature-Programmed Desorption (TPD) analysis of ammonia, desorption amount of ammonia in a region of from 100 to 350° C. is 60% or more of the entire ammonia desorption amount (a region of from 25 to 700° C.); and (3) In thermogravimetry (TG), desorption amount of water is 3% by weight or more of a reference weight in a region of from 32 to 250° C.
Owner:MITSUBISHI CHEM CORP
Method for evaluating flame retardant efficiency of asphalt
InactiveCN103293079AAccurate evaluationFast testWeighing by removing componentMaterial heat developmentBituminous materialsEngineering
The invention discloses a method for evaluating the flame retardant efficiency of asphalt, belonging to the technical field of an asphalt pavement and solving the problem that a method for evaluating the flame retardant efficiency of the asphalt is difficultly accurately quantified by using an existing flame retardant. According to the method, based on the combination of a thermogravimetry-differential thermal analysis synchronous test and a thermal analysis kinetics theoretical equation, the flame retardant efficiency of a flame retardant to the asphalt is quantificationally evaluated. The method comprises the steps of: respectively testing the asphalt and prepared flame-retardant asphalt by adopting a thermogravimetry-differential thermal analyzer to obtain test data such as TGs (Thermal Gravity), DTGs (differential thermogravimetry), DTAs (Differential Thermal Analysis) and char yields; secondly, drawing curves of 1n[g(alpha) / T2] to 1 / T according to the thermal analysis kinetics theoretical equation, and determining reaction mechanism functions g(alpha) in the thermolysis process of the asphalt and the prepared flame-retardant asphalt through linear fitting of a least square method; thirdly, drawing a straight line of the 1n[g(alpha) / T2] to the 1 / T, solving kinetics parameter activation energies E and frequency factors A through a slope and an intercept; finally, comparing the E and the A of the asphalt with the E and the A of the prepared flame-retardant asphalt so as to completely and accurately evaluate the flame-retardant efficiency of the flame retardants with different types and doping quantities to the asphalt.
Owner:NANJING FORESTRY UNIV
Adhesive film, multilayer circuit board, electronic component and semiconductor device
InactiveUS20120156502A1Reliable electrical connectionImprove insulation reliabilityNon-macromolecular adhesive additivesPrinted circuit detailsElectronic componentSemiconductor
Disclosed is an adhesive film in which the adhesive film contains a thermosetting resin (A), a curing agent (B), a compound having flux activity (C) and a film forming resin (D), the minimum melt viscosity of the adhesive film is 0.01 to 10,000 Pa·s, and the adhesive film satisfies the following formula (1) when the exothermic peak temperature of the adhesive film is defined as (a) and the 5% weight loss temperature by thermogravimetry of the adhesive film is defined as (b),(b)−(a)≧100 degrees centigrade (1).
Owner:SUMITOMO BAKELITE CO LTD
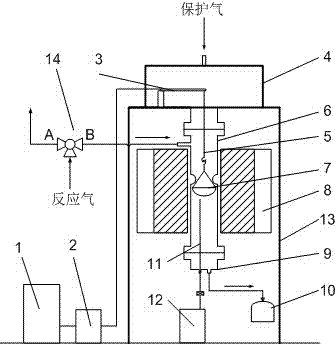
Coal spontaneous-combustion characteristic determining device based on thermogravimetry
ActiveCN105807029ARealize measurementRealize determinationFuel testingVapor phase chromatographyData acquisition
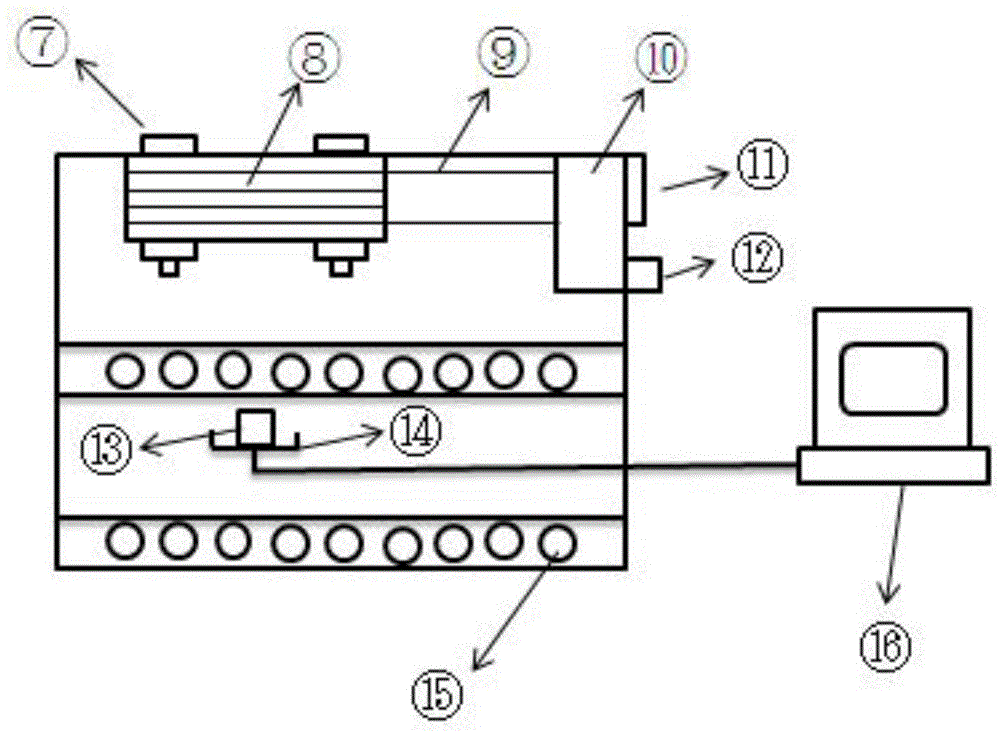
The invention relates to a coal spontaneous-combustion characteristic determining device based on a thermogravimetry.The problem of synchronous measurement of an oxidation temperature rise characteristic curve, mass change quantity and gas production / consumption characteristics of a coal sample under temperature raising programming is effectively solved.High-pressure steel cylinders are connected with a preheating copper pipe through a gas premixing device, the preheating copper pipe is arranged in a coal sample tank support, the coal sample tank support extends out of a box body to be arranged on a balance, a cooling copper pipe is arranged in the top, a coal sample tank is arranged in the coal sample tank support, the preheating copper pipe and the cooling copper pipe are connected with a gas inlet and a gas outlet of the coal sample tank, and the cooling copper pipe is connected to a gas chromatograph.A heating layer is arranged outside the box body, electric heating wires are arranged in the heating layer, a heat preservation layer is arranged outside the heating layer, the electric heating wires are connected with a temperature raising programming controller, a temperature is arranged in the coal sample tank and is connected with a coal sample temperature data acquisition card, and the temperature raising programming controller, the coal sample temperature data acquisition card, the balance and the gas chromatograph are connected with a computer.The coal spontaneous-combustion characteristic determining device is high in automation degree, and test results are reliable.
Owner:HENAN POLYTECHNIC UNIV
Carbodiimide composition with suppressed yellowing, a stabilizer against hydrolysis and a thermoplastic resin composition
ActiveUS7368493B2Improve heat resistanceNo coloring problemOrganic chemistryChemical inhibitorsHeat resistanceAntioxidant
A carbodiimide composition having an improved heat resistance which meets conventional requirements for a carbodiimide compound to be compounded in an ester-group-containing resin or a biodegradable plastic, and exhibiting no coloring problems due to yellowing, and having a superior stabilizing effect agains hydrolysis. The carbodiimide composition comprises a carbodiimide compound (A) and an antioxidant (B), having a 5% weight loss temperature not lower than 250° C. as determined by a thermogravimetric (TG) method, wherein an antioxidant (B) is dispersed and present in the composition by admixing during synthesis of a carbodiimide compound (A), a stabilizer against hydrolysis mainly comprising the carbodiimide composition and a thermoplastic resin composition containing the same.
Owner:NISSHINBO IND INC
Hollow Silica Microparticles, Compositions for Forming Transparent Coating Film Containing the Same, and Substrate Having Transparent Coating Film
InactiveUS20090286070A1Excellent abrasion resistanceGood adhesivenessMaterial nanotechnologyPigmenting treatmentMicroparticleSilicon dioxide
Hollow silica microparticles suppress whitening of a transparent coating film and show excellent abrasion resistance and adhesiveness. The microparticles having the average particle diameter of 5 to 300 nm and the specific surface area of 50 to 1500 m2 / g, and also having an outer shell in which cavities are formed. The microparticles lose the weight by 1.0 W % or more at a temperature range from 200° C. to 500° C. when measured by the thermogravimetry (TG), and the microparticles have a positive DTA peak at the temperature range when measured by the differential thermogravimetric analysis (DTA).
Owner:JGC CATALYSTS & CHEM LTD
Preparation method of superparamagnetic Fe3O4 microspheres
InactiveCN103274477ASimple stepsShort reaction timeMaterial nanotechnologyFerroso-ferric oxidesZeta potentialAlcohol
The invention brings forward a controllable technology for preparing superparamagnetic Fe3O4 microspheres by an amino-terminated alcohol and glycol combined double-solvent method. According to the technology, proportions of the two solvents can be changed to adjust the dimension of the microsphres and particle size of particles which form the microspheres. The diameter of the microspheres can be observed through a transmission electron microscope; the particle size of the microspheres can be measured by x-ray diffraction; the microspheres which undergo different surface modifications are prepared by changing a protective agent; surface components can be measured through infrared, zeta potential, thermogravimetry and the like; and magnetic property is measured by a sample vibration magnetometer.
Owner:SANMING UNIV
Magnetic material curie temperature measuring method based on thermogravimetry changes
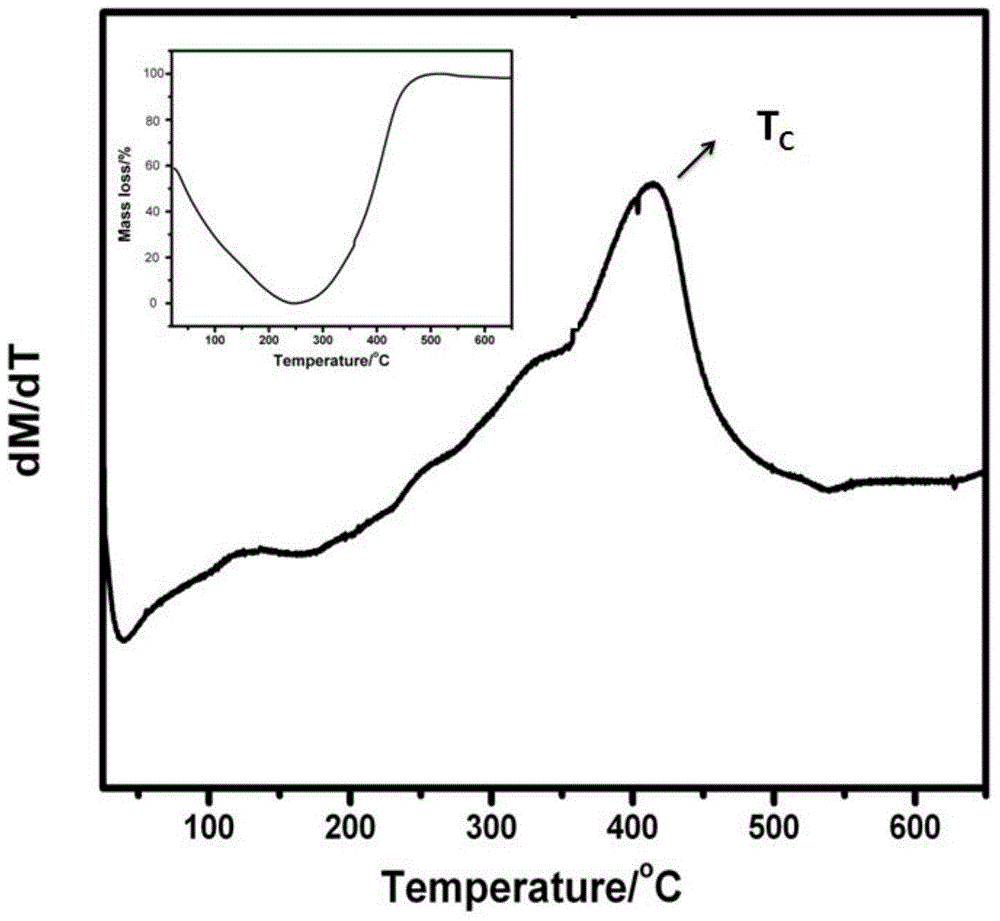
ActiveCN104568209ASolve fever problemSignificant change in thermogravimetricThermometers using electric/magnetic elementsUsing electrical meansUniform fieldCurie temperature
The invention belongs to the technical field of the thermomagnetic measurement of magnetic materials, and relates to a magnetic material curie temperature measuring method based on thermogravimetry changes. Through measuring the changing relationship between balance apparent weight and temperature under the effect of a low field intensity applied magnetic field, the curie temperature of a magnetic substance is obtained. The suction effect of an electromagnet to the magnetic substance is related to magnetic field intensity and also relies on the gradient changes of the magnetic field intensity, therefore the obvious thermogravimetry changes can be obtained under a non-uniform field intensity applied magnetic field with relatively lower field intensity, and the changes can be easily measured by a high-precision analytical balance. According to the magnetic material curie temperature measuring method based on the thermogravimetry changes, the obvious thermogravimetry changes can be obtained by using the non-uniform field intensity applied magnetic field with the relatively lower field intensity, and then the curie temperature of the magnetic substance can be measured; the problem of coil heating caused by the fact that a high-intensity magnetic field needs to be loaded when materials with weaker magnetism are measured by using a magnetic-thermo curve method is avoided; the method is economical and convenient, and is easy to popularize.
Owner:DALIAN UNIV OF TECH
Method for predicting pyrolysis process of environment-friendly flame-retarding asphalt
InactiveCN103471956AAccurate predictionAccurate service lifeWeighing by removing componentPredictive methodsBituminous materials
Owner:NANJING FORESTRY UNIV
Preparation method for anti-oxidant silica-based ceramic coating with wide temperature range for carbon/carbon composite
ActiveCN102745998AOvercoming poor compactnessOvercoming the thermal expansion mismatch between inner and outer layersCarbon compositesNanowire
The invention relates to a preparation method for an anti-oxidant silica-based ceramic coating with a wide temperature range for a carbon / carbon composite. The preparation method has the technical characteristics of preparing a silica-based ceramic coating toughened by SiC nanowires on the surface of the carbon / carbon composite by employing a combination of an embedding impregnation method and an in-situ synthesis method. The preparation method overcomes problems of poor compactness and the mismatch of thermal expansion between an inside layer and an outside layer of the silica-based ceramic coating toughened by SiC nanowires prepared by the background technology. The prepared coating can realize anti-oxidant protection in the wide temperature range for the C / C composite. The results show that the C / C composite with the prepared coating keeps a weight gain state all the time in a thermogravimetry testing process at the temperature of 1500 DEG C, and the maximum weight gain rate id 1.078%-1.156%.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Method for measuring content of free magnesium oxide in steel slag
The invention relates to a method for measuring content of free magnesium oxide in steel slag. The method comprises the following steps: firstly, grinding steel slag to be measured into steel slag powder, putting the steel slag powder into a pressure container for pressure evaporation so as to sufficiently hydrate magnesium oxide in the steel slag powder for completely converting into magnesium hydrate, subsequently drying to be constant weight, grinding the steel slag powder and uniformly stirring, subsequently respectively putting the steel slag before and after the pressure evaporation into a thermogravimetry tester, and calculating the percentage W of the content of free magnesium oxide in steel slag according to the difference C of mass loss percentages of the steel slag before and after the pressure evaporation. By adopting the method, the content of free magnesium oxide can be accurately measured, and the operation is convenient.
Owner:CENT RES INST OF BUILDING & CONSTR CO LTD MCC GRP +1
Stable hydrochloric acid Ivabradine II crystal form and preparation method
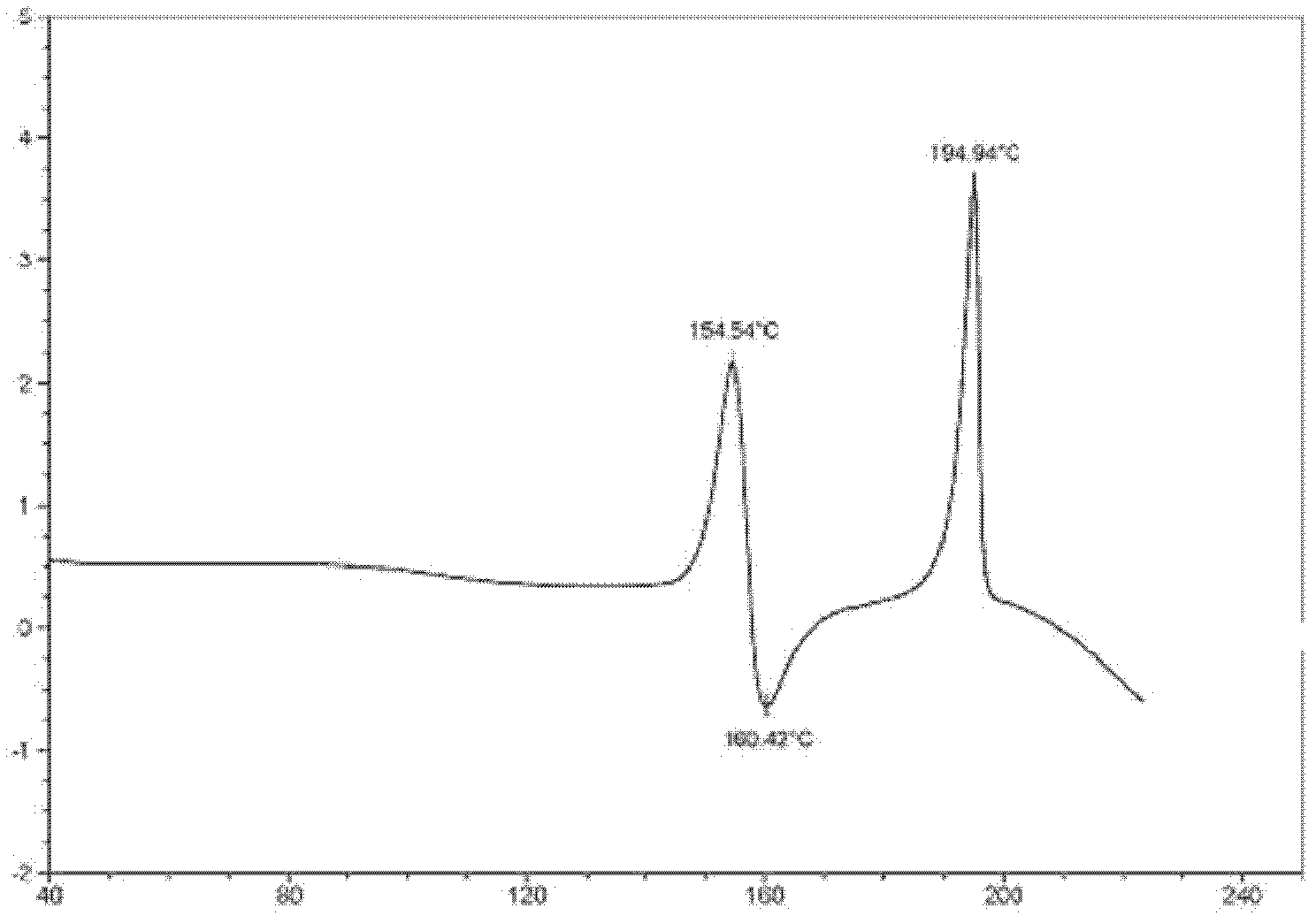
ActiveCN103183639AEasy to preparePromote environmental protectionOrganic chemistrySpace groupDecomposition
The invention provides a stable hydrochloric acid Ivabradine II crystal form, a thermogravimetry spectrogram shows that each part of hydrochloric acid Ivabradine molecule contains 0.5 parts of crystal water. The differential scanning calorimetry (DSC) spectrum diagram shows that a large endothermic peak is generated at -154 DEG C, a heat release peak is generated at -160 DEG C, wherein the fusion decomposition temperature is 194 DEG C (summit value), wherein the hydrochloric acid Ivabradine II crystal form is a monoclinic system, its space group is P21, the cell parameter comprises: a=5.48740(10)Angstrom, b=43.4767(7)Angstrom, c=11.4892(2)Angstrom, beta= 98.144 (2) degrees, and a crystal cell volume is 2713.38(8)Angstrom<3>. A characteristic diffraction spectral line of its X-powder diffraction describes hydrochloric acid Ivabradine new crystal form as II crystal form. The Ivabradine new crystal form enables difficult water absorption and deliquescence, and has the advantages of good stability and convenient storage. The preparation method is simple and easy to be carried out, a solvent with high boiling point and large toxicity is not used, the preparation method is in favor of environmental protection, is suitable for industrial production, and has large application value.
Owner:ZHEJIANG JINGXIN PHARMA +1
Method for determining quality stability of cigarette paper
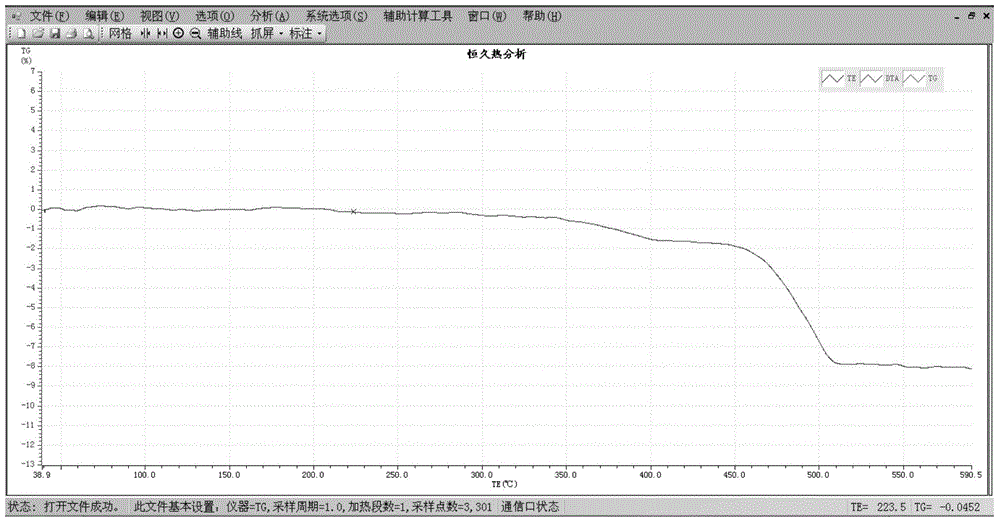
ActiveCN106979904ALess quantityShort analysis timeWeighing by removing componentEngineeringThermogravimetric analysis
The invention discloses a method for determining the quality stability of the cigarette paper. The quality stability of cigarette papers in different batches can be accurately determined by using thermogravimetry (TGA) through comparing the discretivity of the thermogravimetric curve (TG) of the cigarette papers with the same brand and specification and in different batches. The method has the advantages of small amount of needed samples, short analysis time, low cost, simplicity in operation, and accurate experimental result.
Owner:CHINA TOBACCO ANHUI IND CO LTD
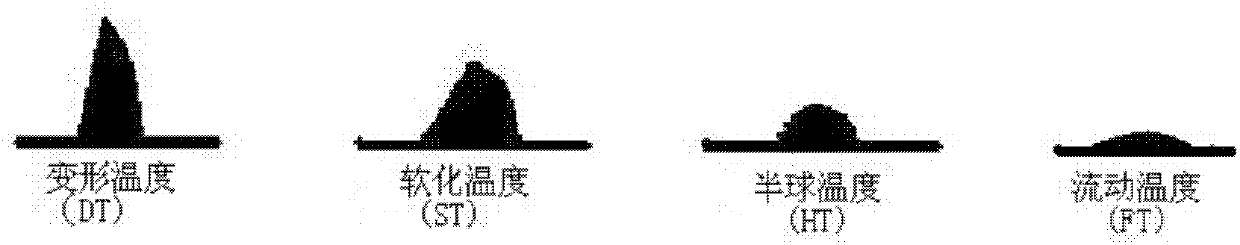
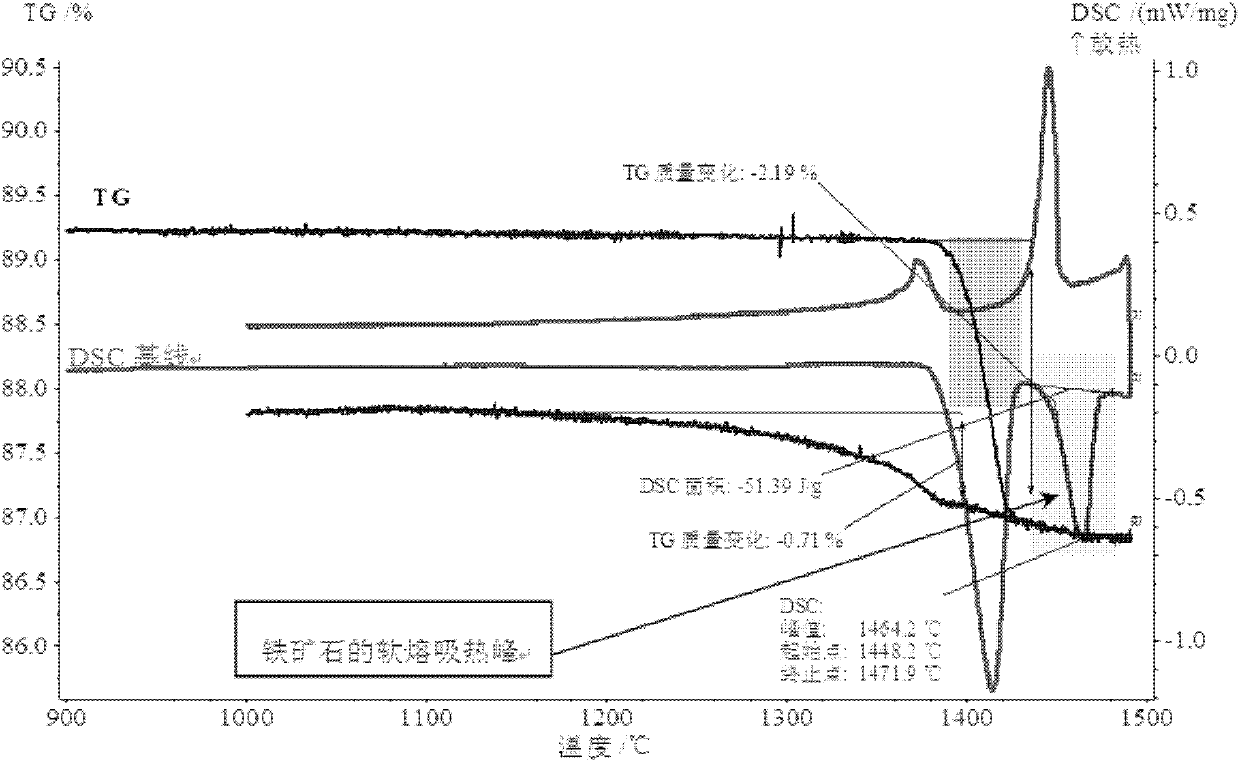
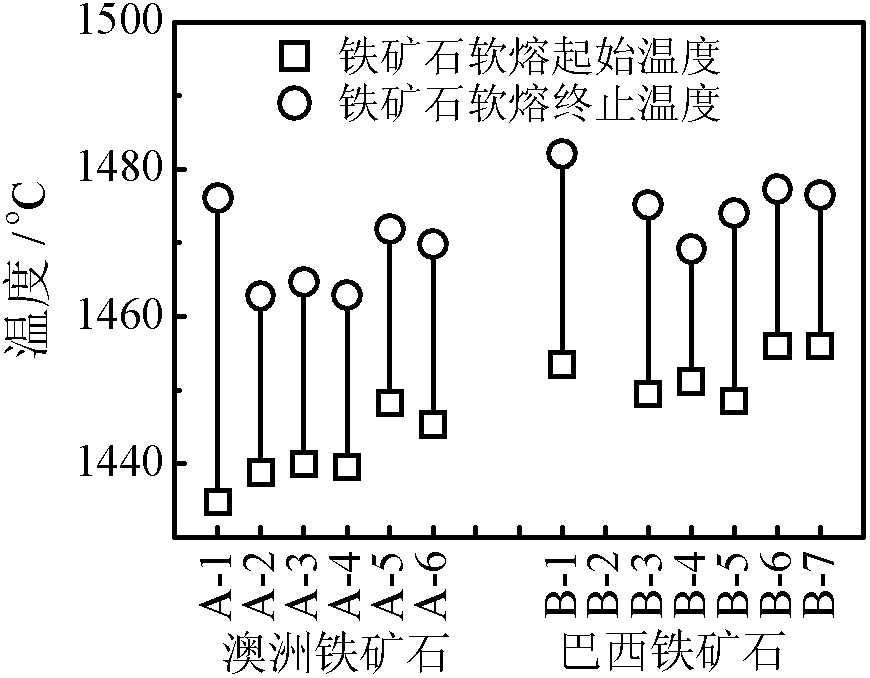
Analytical method for iron ore high-temperature soft melting characteristics
InactiveCN102890098ASolve the problem of high temperature reflow characteristics detectionThe process is simple and convenientMaterial heat developmentInvestigating phase/state changeProcess optimizationAnalysis method
The invention relates to an analytical method for iron ore high-temperature soft melting characteristics. The analytical method is based on TG-DSC (Thermogravimetry-Differential Scanning Calorimetry) thermoanalysis technology, has simple process and accurate detection result, can effectively solve the problem of detecting the high-temperature soft melting characteristics of the iron ore, can accurately reflect the characteristic parameters such as soft melting starting temperature, final temperature and temperature range and the like of the iron ore, at the same time, can represent important information such as the amount of liquid phase as well as the relationship between the amount of liquid phase and the iron ore composition in the process of soft melting, thereby providing important basic data for process optimization in the processes of ore blending and sintering.
Owner:BAOSHAN IRON & STEEL CO LTD
Heat ray shielding laminated glass and manufacturing method for heat ray shielding laminated glass
InactiveUS20160271910A1Excellent Adhesive PropertiesImprove flatnessLaminationLamination apparatusThermal radiationMoisture
An object of the present invention is to provide a heat ray shielding laminated glass which has excellent flatness and adhesion between a glass substrate and a heat ray shielding film unit, and has a reduced glass scattering rate even when the glass substrate is damaged by an external impact, and a manufacturing method therefor.The heat ray shielding laminated glass of the present invention is a heat ray shielding laminated glass which is formed by press bonding of a pair of glass substrates on both surfaces of a heat ray shielding film unit A, which has a heat ray shielding film having at least one heat ray shielding layer on a transparent resin film and at least one adhesive layer, the heat ray shielding laminated glass being characterized in that the heat ray shielding film unit A has an average moisture content 1.0% by mass or less as determined by TG-DTA (simultaneous measurement of thermogravimetry•differential thermal analysis).
Owner:KONICA MINOLTA INC
Silver coordination polymer and preparing method and application thereof
InactiveCN105646550AImprove thermal stabilitySpecial fluorescent propertiesGroup 1/11 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsFluorescenceSynthesis methods
The invention discloses a silver coordination polymer. The chemical formula of the silver coordination polymer is C24H20AgN4O2, 4,4'-di(parazole-1-methyl)-biphenyl is taken as the major ligand, terephthalic acid is taken as the auxiliary ligand, distilled water is taken as the solvent, the microwave heating reflux synthesis method is adopted, the first binode two-dimensional '4,4L10' topological network structure is formed, sufficient representation of the structure is achieved through elemental analysis, thermogravimetry and a single crystal X-ray diffractometer, and the luminescent property and degradation property on methyl orange dye of the structure are researched in detail too. The preparing process is simple, the yield and purity of crystals are high, high thermal stability and special luminescent property are realized, organic pollutants can be degraded through Fenton-like reaction, degradation efficiency is high, and the silver coordination polymer can serve as a catalyst and has great application prospects in the field of luminescent materials and environment protection.
Owner:HUAIHAI INST OF TECH
Titanium oxide and photocatalyst
InactiveUS7011808B2Improve photocatalytic activityCellulose coatingsCatalyst activation/preparationMass numberSpectroscopy
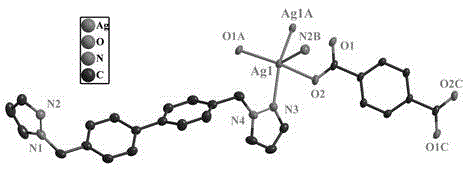
A titanium oxide showing sufficiently high photocatalytic activities by irradiation of visible light is provided. Using the titanium oxide, an excellent photocatalyst and photocatalyst coating composition are also provided. The titanium oxide has a selected ion chromatogram in which an evolution gas having 28 of a ratio of mass number to electric charge quantity exhibits at least one peak at about 600° C. or higher, the selected ion chromatogram being measured in a thermogravimetry-mass-spectroscopy.
Owner:SUMITOMO CHEM CO LTD
Isothermal thermogravimetry apparatus
InactiveCN106092803ARealize constant temperature thermogravimetric analysis functionStable temperatureMaterial weighingElectricityElectric arc furnace
The invention discloses an isothermal thermogravimetry apparatus. The apparatus comprises an electric furnace, a gas preheater, a tray, a walking device, a weighing device and a temperature controller, a coiled tube is arranged in the gas preheater, the front end of the coiled tube is provided with a gas flow meter, the tail end of the coiled tube is introduced to the inner side of the electric furnace, the weighing device is fixedly arranged on the walking device, the front end of the support of the weighing device is fixedly provided with the tray, and the temperature controller is used for controlling the gas preheater and the temperature in the electric furnace. The electric furnace used in the invention is a high-temperature electric furnace, and realizes heating through a high-temperature electric heating rod, the temperature controller controls the heating rate of the electric furnace, can realize the temperature programming process, and also can keep the temperature unchanged at a preset value, and the hearth of the electric furnace is made of an anticorrosive high temperature material and can resist SO2, NO, HCl and other corrosive gases, so the isothermal thermogravimetry apparatus can realize the isothermal thermogravimetry function, and also can realize temperature programming thermogravimetry of traditional thermobalances.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
Detection analysis method for asphalt volatile organic compound
InactiveCN103900926AEmission reductionWeighing by removing componentMaterial analysis by electric/magnetic meansTemperature controlMass Spectrometry-Mass Spectrometry
The invention discloses a detection analysis method for an asphalt volatile organic compound (VOC). The detection analysis method adopts a combination manner of thermogravimetry-mass spectrometry (TG-MS) to directly carry out mass spectrometry on volatile gas in the procedure temperature controlling heating process during the operation of carrying out a thermal weight loss test on asphalt. The mass of an asphalt sample is 5-10mg. The program temperature control in the thermal weight loss testing process is raised to 250 DEG C at the speed of 10 DEG C / minute. The mass spectrometry adopts molecular weight scanning with the molecular weight from 0 to 300. A TG-MS coupled technology can be used for simultaneously determining the quality change of the asphalt and qualitatively and quantitatively analyzing the VOC in a heating state of the asphalt material and the escaping quantities of the VOCs of the different asphalt can be balanced; the relatively direct reference is provided for an asphalt VOC inhibitor researcher through determining VOC components, and the inhibition condition an asphalt VOC inhibitor of on the VOC can also be directly responded, so that the emission of the asphalt VOC is reduced.
Owner:WUHAN UNIV OF TECH
Thermal analyzer with gas mixing chamber
ActiveUS7104680B2Accurate measurementWeighing by removing componentMaterial thermal coefficient of expansionHigh humidityWater vapor
In a thermomechanical measuring device and a thermogravimetry device, partition walls are provided in two sections such that two kinds of atmospheric gasses, which have passed a sample chamber and a detector chamber, respectively, do not flow back, and a thermally insulated gas mixing chamber is manufactured anew in the middle of the sample chamber and the detector chamber to make it possible to dilute a reactive gas and a water vapor gas having a high partial pressure. Consequently, it is possible to prevent moisture concentration to reduce an influence of water drops even in a high temperature and high humidity state at the time of humidity control and measurement.
Owner:HITACHI HIGH TECH SCI CORP
Carbon material for negative electrode for lithium ion secondary battery, manufacturing process therefor and use thereof
InactiveCN104685680AImprove initial efficiencyImprove discharge capacityGraphiteNegative electrodesLithiumX-ray
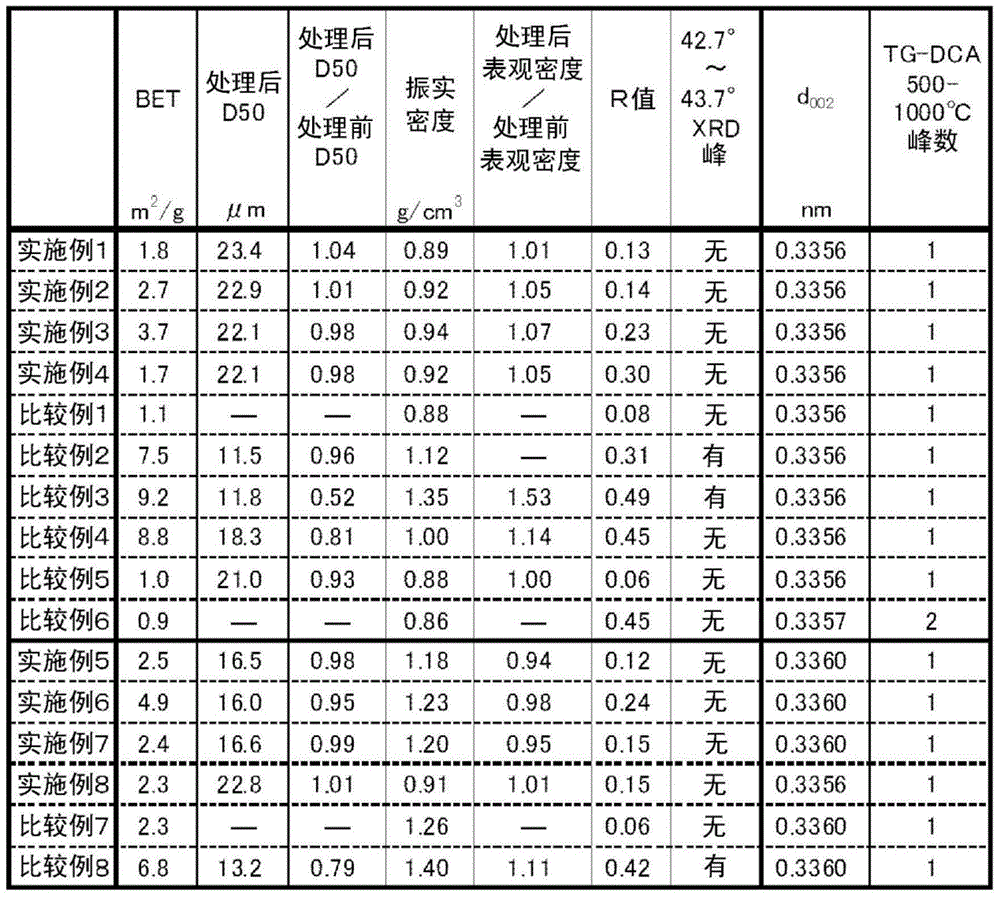
A carbon material for a negative electrode for a lithium ion secondary battery, having a specific surface area of 1.5 to 6.5m2 / g, a tap density of 0.5 to 1.3g / cm3, and a Raman R value of 0.1 to 0.4. Further, this carbon material exhibits no diffraction peak in a diffraction angle range of 42.7 to 43.7 degrees in X-ray diffraction, has a d002 of 0.337nm or less, and exhibits at most one peak within a region of 500 to lower than 1000ºC in thermogravimetry-differential thermal analysis.
Owner:RESONAC HOLDINGS CORPORATION
Diesel engine grading particle pyrolysis activation energy quantitative evaluation method
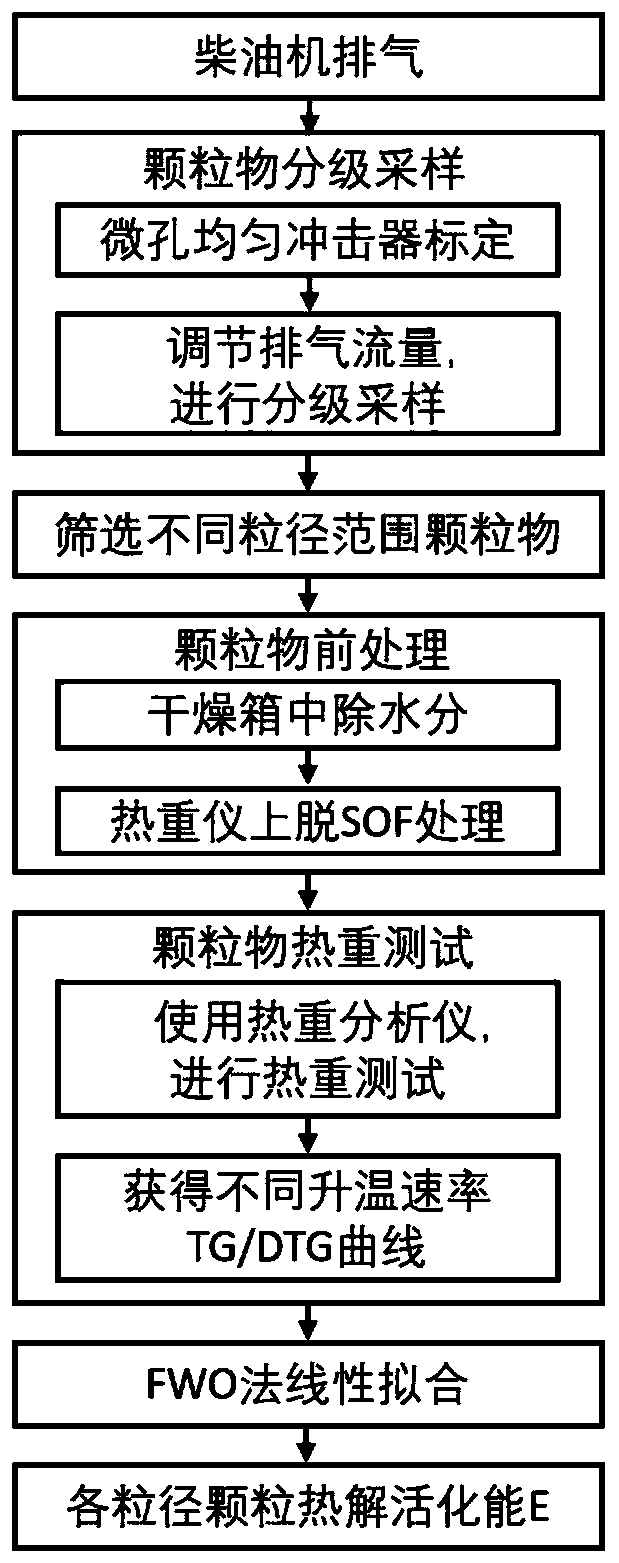
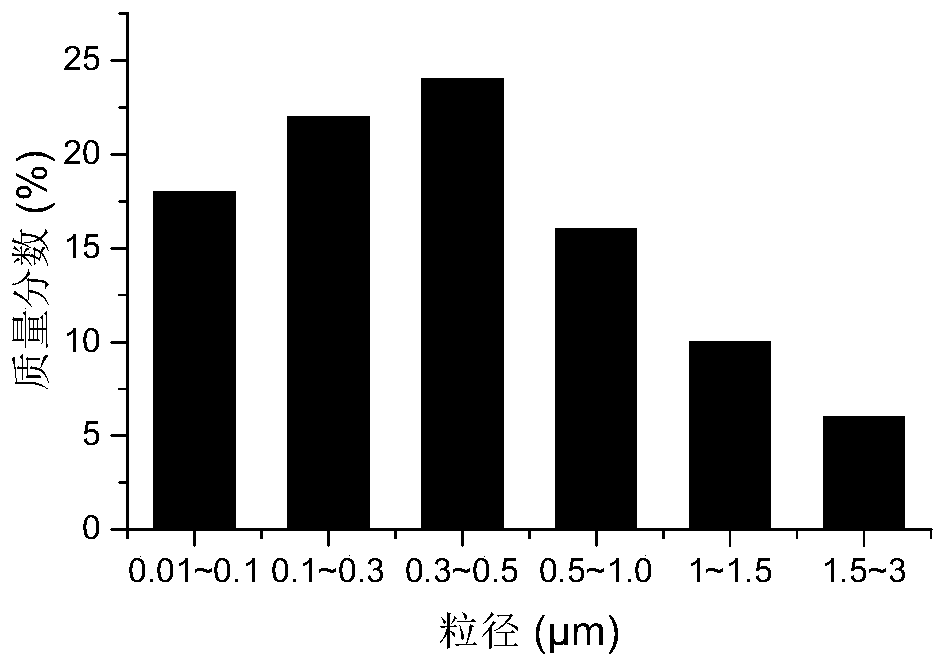
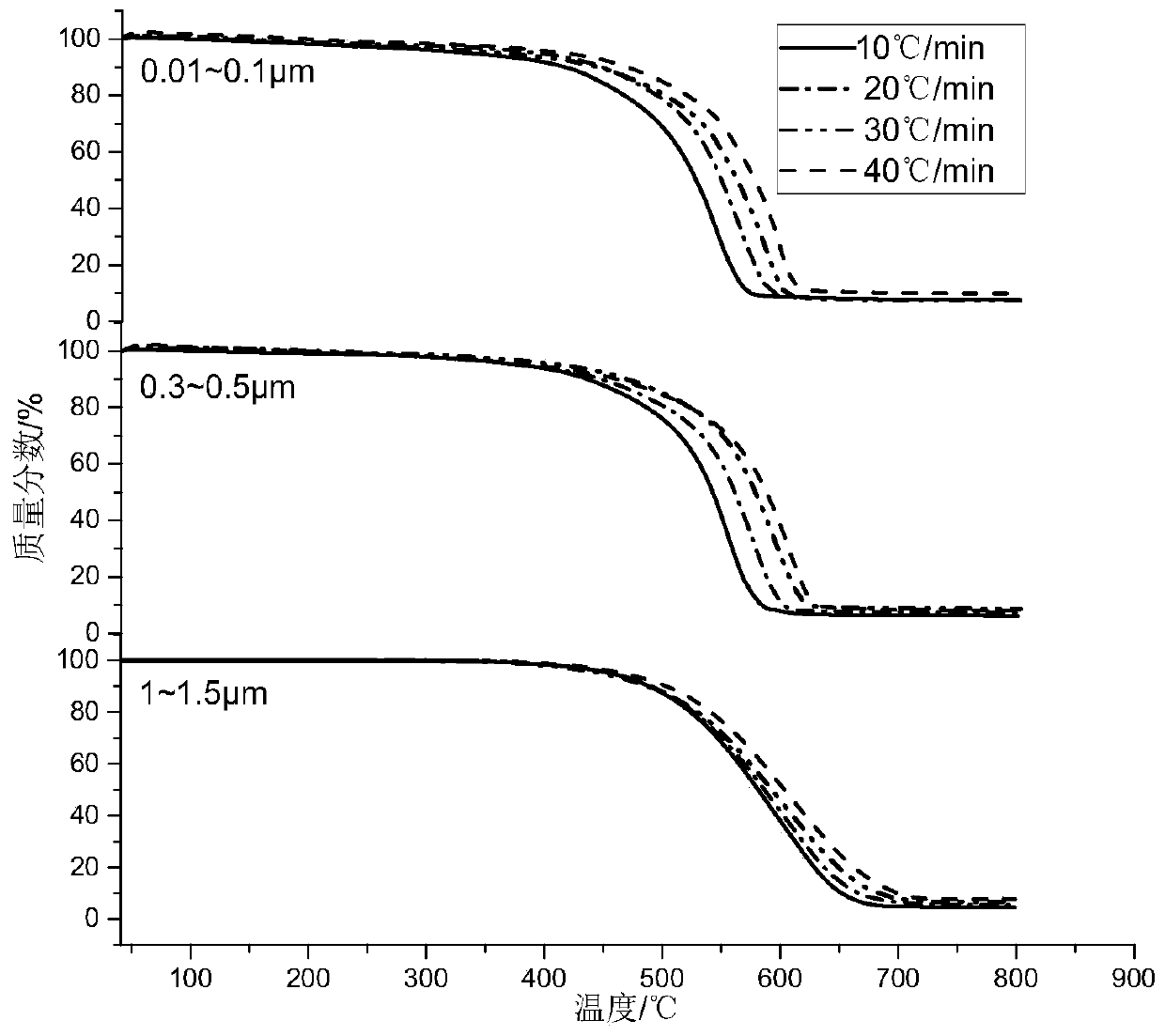
PendingCN110793891AAccurate Activation Energy ParametersEffectively eliminate test interference factorsWeighing by removing componentParticle size analysisParticulatesDieseling
The invention discloses a diesel engine grading particle pyrolysis activation energy quantitative evaluation method, and relates to the technical field of the diesel engine grading particle emission.The method comprises four steps of grading of particulate matters, pretreatment of particulate matters, thermogravimetry test on the particulate matters in different particle size ranges, and mathematical calculation analysis on acquired thermogravimetry data by adopting a FWO method formula. A micropore uniform deposition impactor and a thermogravimetry analyzer measurement device are used for collecting and treating the particulate matters to acquire a TG / DTG curve of the particulate matters in different particle size ranges, and the final pyrolysis activation energy values of the particulate matters in different particle size ranges can be obtained by performing linear fitting manner on the obtained data. Through the method disclosed by the invention, the oxidation activation energy ofthe particulate matters in different particle sizes of the to-be-tested diesel engine can be quickly and accurately acquired, thereby providing effective parameters for the regenerative design of a DPF.
Owner:JIANGSU UNIV
Micro device and modeling method for studying gas-solid intrinsic chemical reaction kinetics
ActiveCN104502535AAdequate responseEasy to observeMaterial analysisChemical reaction kineticsData acquisition
The invention discloses a micro device for studying gas-solid intrinsic chemical reaction kinetics. The micro device comprises a weighing component, a reactor component, a bracket and a three-directional switching valve, wherein the weighing component comprises a computer, a data acquirer, a strain gage, a glass protecting cover and a metal wire hook; the reactor component comprises a hollow quartz glass reactor, a metal hoisting basket, a heating furnace, a hollow quartz connecting part, a gas collection bag, a thermocouple and a temperature control box. The invention further provides a modeling method using the micro device. The modeling method comprises the following steps: on the premise that the influence caused by flowing such as internal and external diffusion is eliminated, firstly, confirming the conversion rate of a gas reactant and the yield of a multi-component gas product, and finally with the combination of change of the mass of solid test samples in different time periods, acquiring a gas-solid intrinsic chemical reaction kinetics model. By adopting the micro device disclosed by the invention, the problems that a conventional thermogravimetry device is small in test sample amount, a product cannot be quantified, the influence caused by internal and external diffusion is hard to eliminate, and the like are overcome, and a novel method for studying the gas-solid intrinsic chemical reaction kinetics is developed.
Owner:SOUTHEAST UNIV
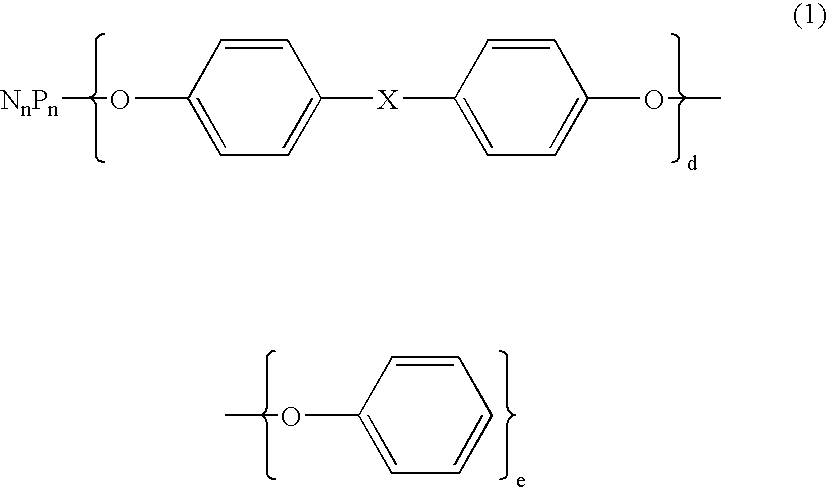
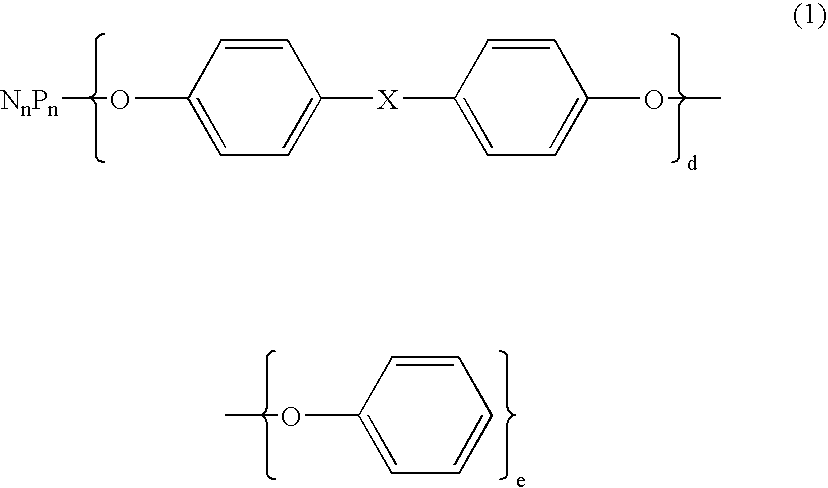
Flame retardant and an epoxy resin composition comprising the same for encapsulating semiconductor devices
ActiveUS20060223913A1Without compromising reliabilityPrevent hydrolysis of the phosphazenePlastic/resin/waxes insulatorsGroup 5/15 element organic compoundsEpoxyDevice material
A flame retardant comprising an inorganic porous fine particle, a phosphazene compound represented by the following average compositional formula (1) wherein X is selected from the group consisting of a single bond, CH2, C(CH3)2, SO2, S, O, and O(CO)O, n is an integer of from 3 to 1000, d and e are numbers with 2d+e=2n, said phosphazene compound being supported on said inorganic porous fine particle, and a resin layer coating said inorganic porous fine particle with the phosphazene compound supported thereon, said resin thermally decomposing to lose weight by 10% at a temperature of from 300° C. to 500° C. as measured by thermogravimetry in the air at a heating rate of 10° C. / min.
Owner:SHIN ETSU CHEM IND CO LTD
Halogen-free resin composition, insulated wire and cable
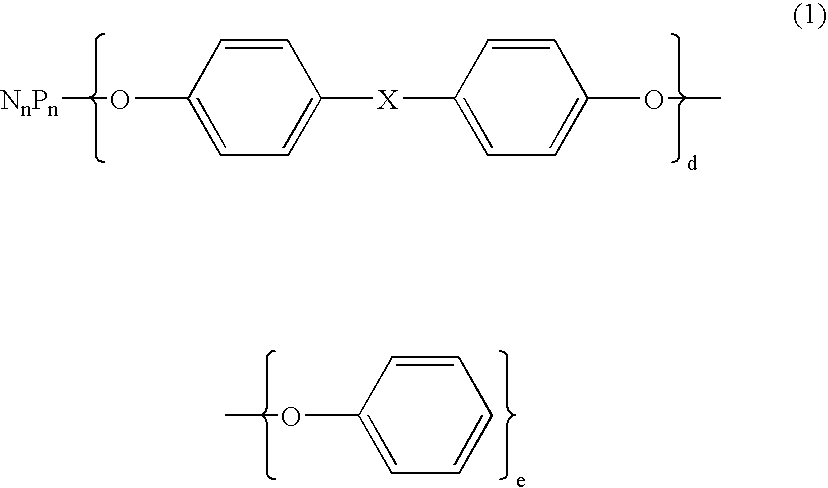
InactiveUS20150060107A1Avoid spreadingPlastic/resin/waxes insulatorsInsulated cablesWeight changeEngineering plastic
A halogen-free resin composition includes an engineering plastic as a main component including an aromatic ring. A thermal weight-change rate measured by a thermogravimetry (under conditions that a dry air as a purge gas is introduced and that heating is conducted from 40° C. at a temperature rise rate of 10° C. / min) is not less than −60% when it is 430° C.
Owner:HITACHI METALS LTD
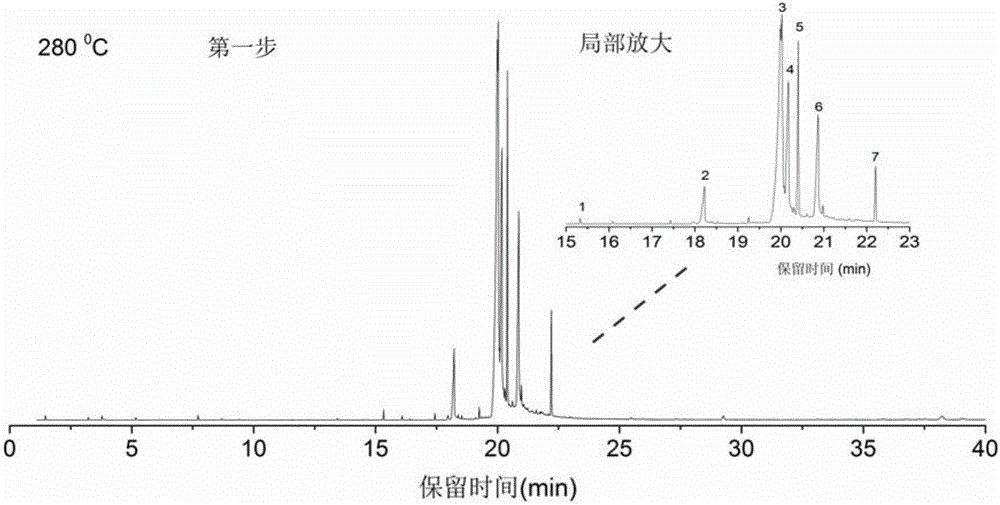
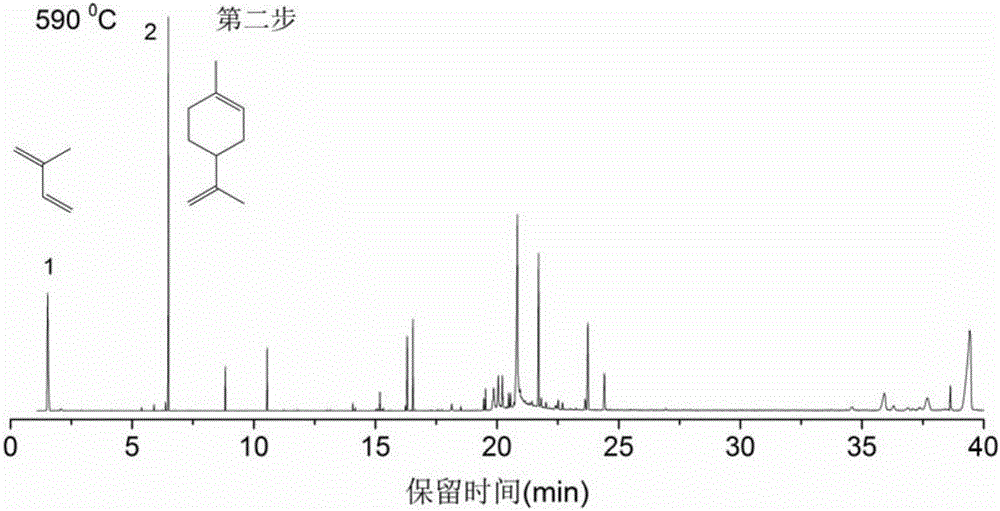
Method for identifying structure and composition of non-rubber component in natural rubber through thermogravimetry-PGC (pyrolysis gas chromatography)-MS (mass spectrum)
InactiveCN107525871ARich application of basic theoryQuick analysisComponent separationMaterial weighingChromatographic columnEvaluation system
The invention discloses a method for identifying a structure and composition of a non-rubber component in natural rubber through thermogravimetry-PGC (pyrolysis gas chromatography)-MS (mass spectrum). The method comprises the following steps: (1) concentrating and drying the non-rubber component obtained from the natural rubber; (2) making a thermogravimetry detection analysis on a sample obtained after drying; (3) analyzing the composition of the sample with a step-by-step cracking method according to a result of the thermogravimetry detection analysis, effectively separating a split product in each step by using a chromatographic column, obtaining corresponding molecular structure information of the split product by using mass spectrum search and primarily judging composition characteristics of substances in the original sample according to the strong and weak degree of signal peaks in a total ion chromatorgraphy. Through application of the method disclosed by the invention, the molecular structure and the composition of the non-rubber component in the natural rubber can be analyzed quickly and accurately, the basic application theory of the natural rubber is enriched, and the technical guidance is provided for perfection of planting, processing and quality evaluation systems of the natural rubber and product production.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com