Stable hydrochloric acid Ivabradine II crystal form and preparation method
A technology of ivabradine hydrochloride and crystal form, which is applied in the field of medicinal chemistry and can solve problems such as easy discoloration, poor fluidity, and unfavorable preparation processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Add 1 g of ivabradine hydrochloride into 60 ml of acetone solution, stir and heat up to reflux, add 0.2 mL of distilled water dropwise until the drug is completely dissolved, and continue to reflux for 30 minutes. Natural cooling and crystallization, the crystallization stopped when the temperature dropped to 55°C, suction filtration, and drying at 80°C to obtain 0.78 g of Ivabradine Hydrochloride Form II.
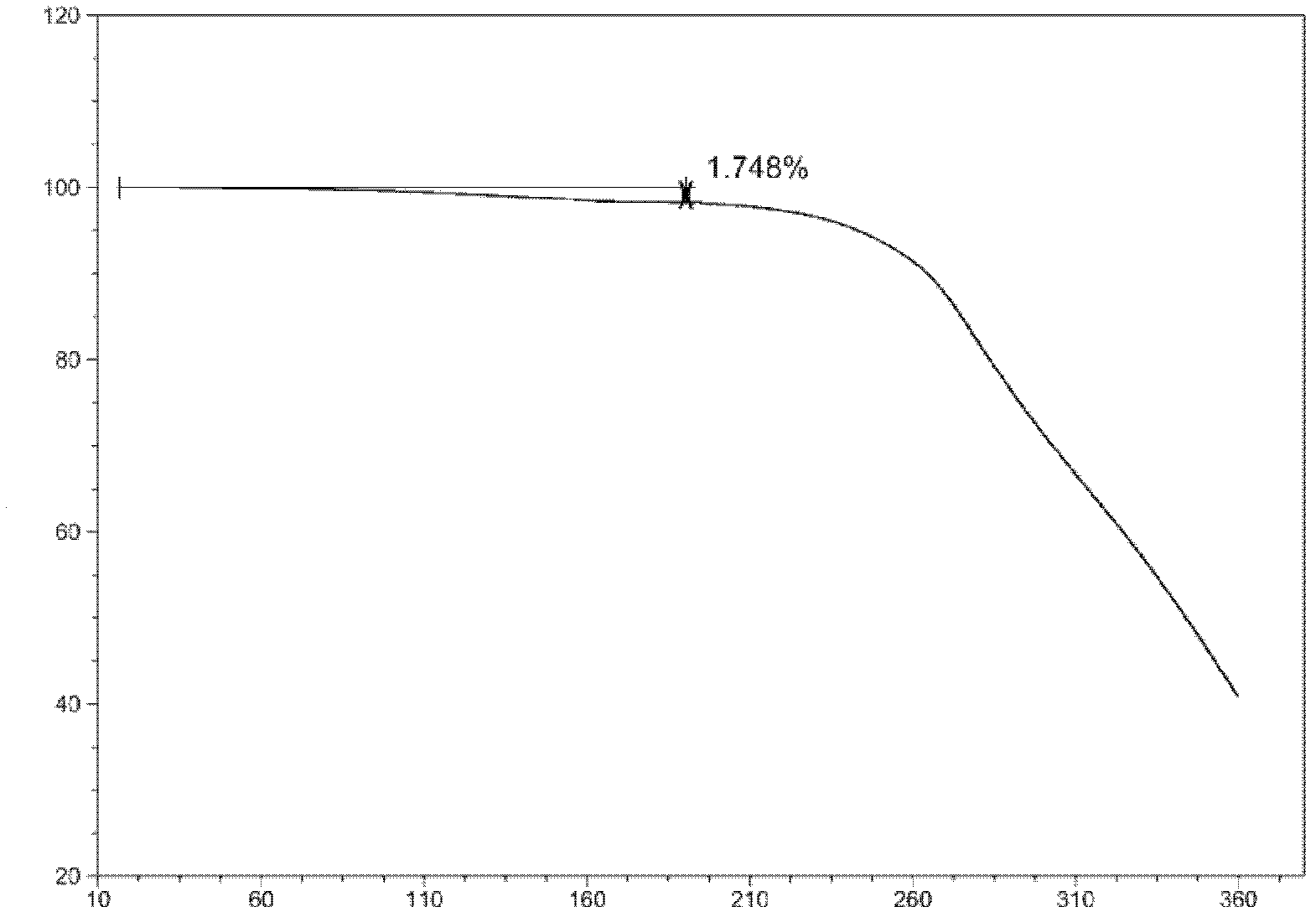
[0086] Thermogravimetric (TG) curve ( figure 1 ) shows that in the range from room temperature to 150°C, the weight loss rate is 1.7%, indicating that the structure contains 0.5 parts of crystal water;
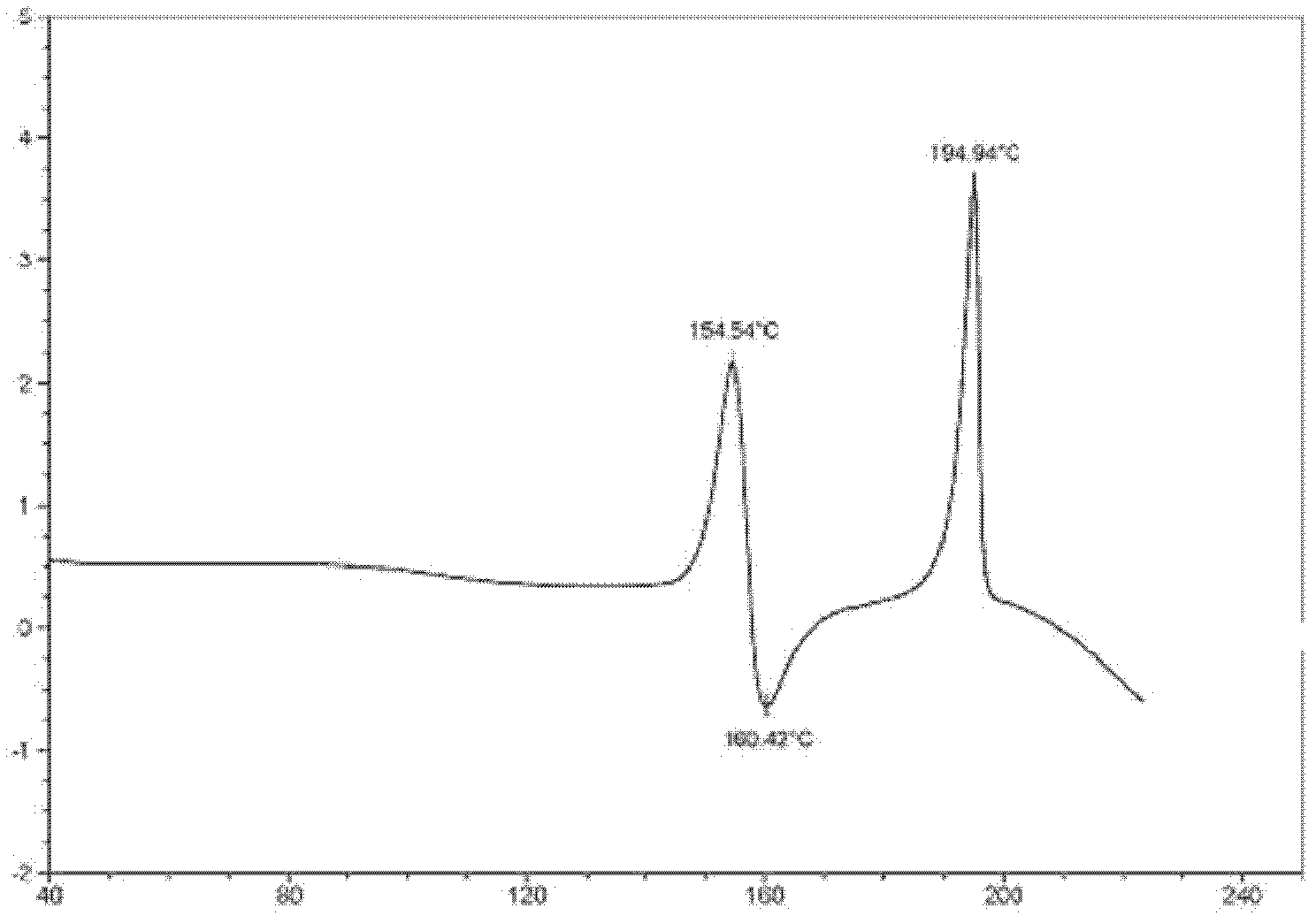
[0087] Differential thermal analysis (DSC) curve ( figure 2 ) (~154°C has a large endothermic peak; ~160°C has an exothermic peak, and the melting decomposition temperature is 194°C (peak value))
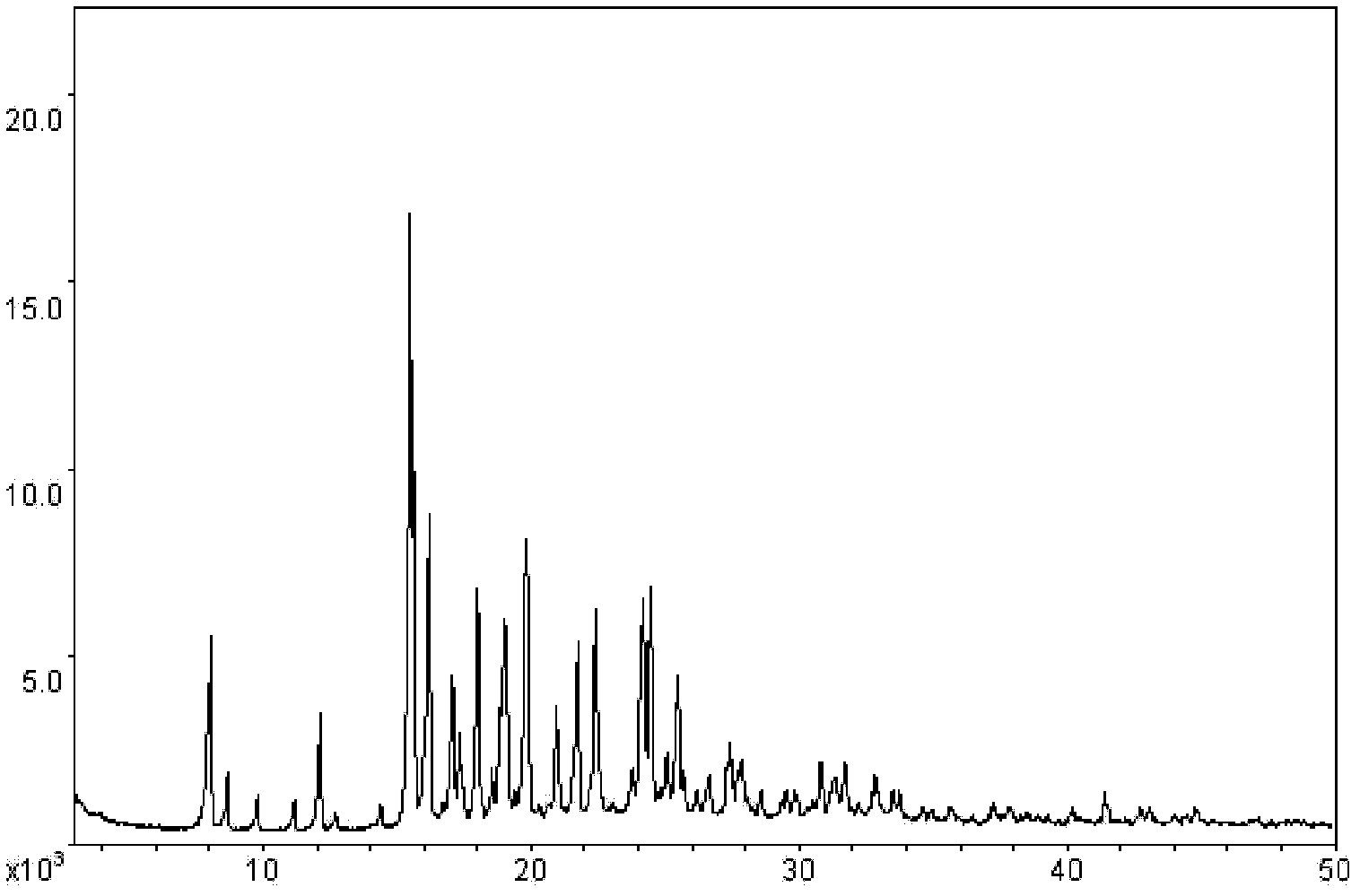
[0088] X-ray powder diffraction spectrum ( image 3 )(Table 1)
[0089] Infrared spectra( Figure 4 ) (at 3464cm -1 , 2932cm -1 , 1645cm -1 , 1520cm -1 , 1459cm...
Embodiment 2
[0095] Add 5 grams of ivabradine hydrochloride to 250 milliliters of tetrahydrofuran and ethyl acetate in a mixed solvent (v / v=2:1 of the ratio of tetrahydrofuran and ethyl acetate), heat to reflux (65-73 ° C), stir After 30 minutes, slowly add 4.5 ml of distilled water dropwise, continue to stir until it is completely dissolved, then cool down naturally to crystallize, stop the crystallization at 55°C, filter with suction, and dry at 80°C to obtain 3.5 grams of Ivabradine Hydrochloride Form II .
[0096] X-ray powder diffraction spectrum ( Figure 11 )
Embodiment 3
[0098]10 grams of ivabradine hydrochloride was added to a mixed solvent of 500 milliliters of tetrahydrofuran and ethyl acetate (v / v=2:1 of the ratio of tetrahydrofuran and ethyl acetate), heated to reflux (65-73 ° C), stirred for 30 Minutes later, slowly add 8.8 milliliters of distilled water dropwise, continue to stir until completely dissolved, naturally cool down to crystallize, stop crystallization at 50°C, filter with suction, and dry at 80°C to obtain 8 grams of ivabradine hydrochloride II crystal form .
[0099] X-ray powder diffraction spectrum ( Figure 12 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com