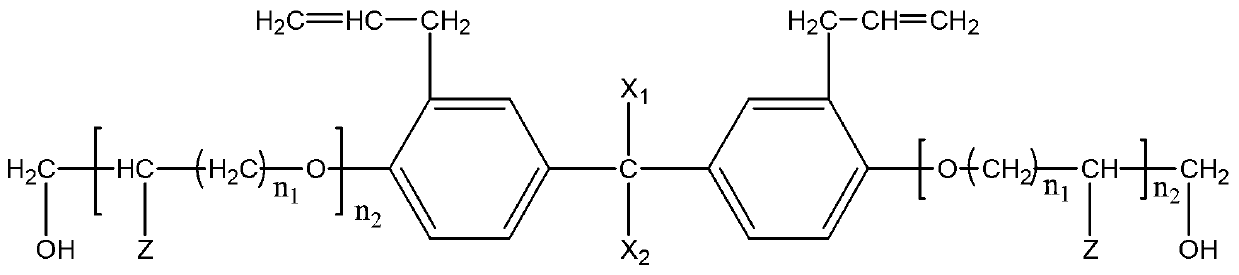
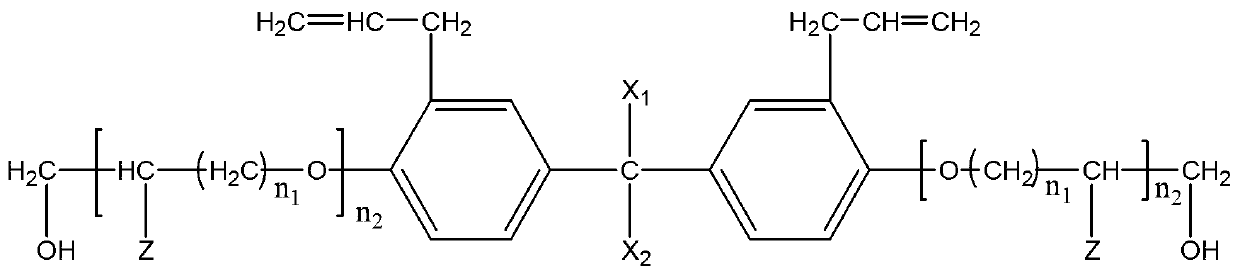
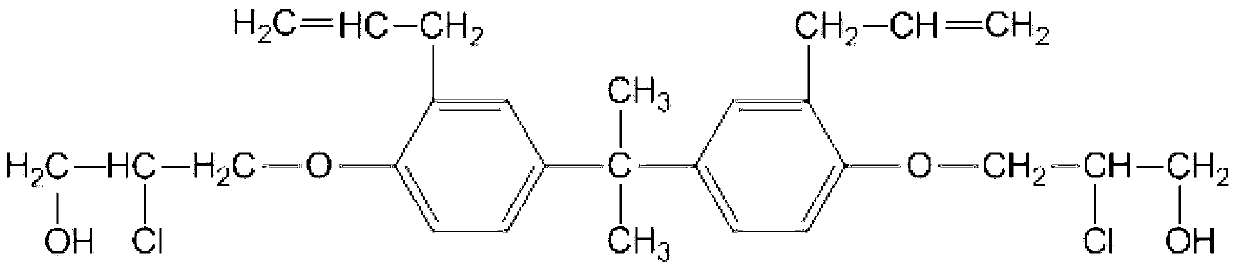
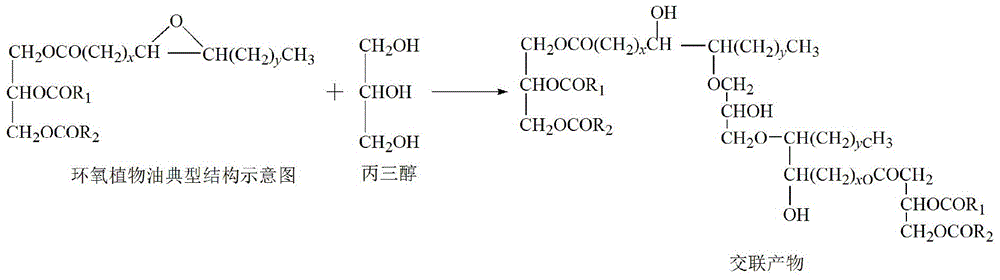
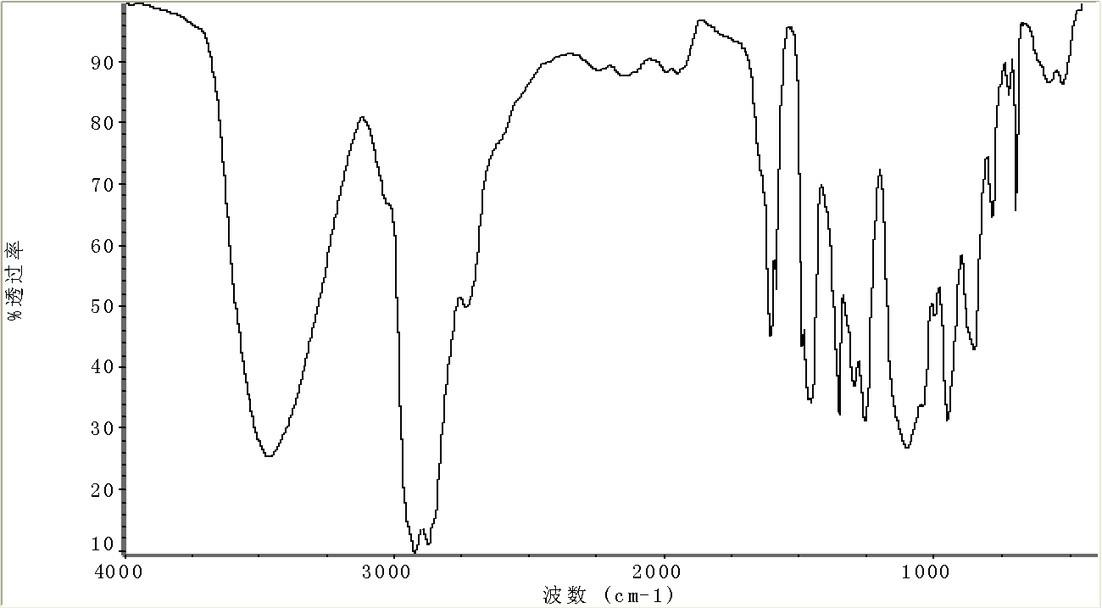
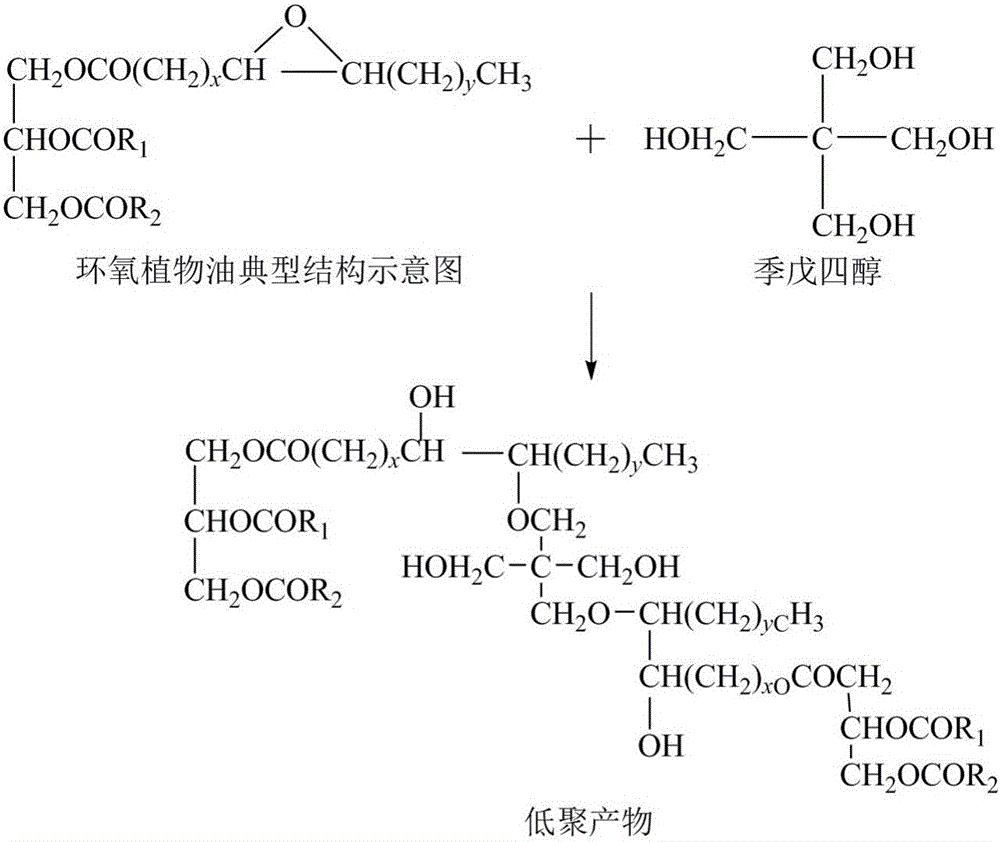
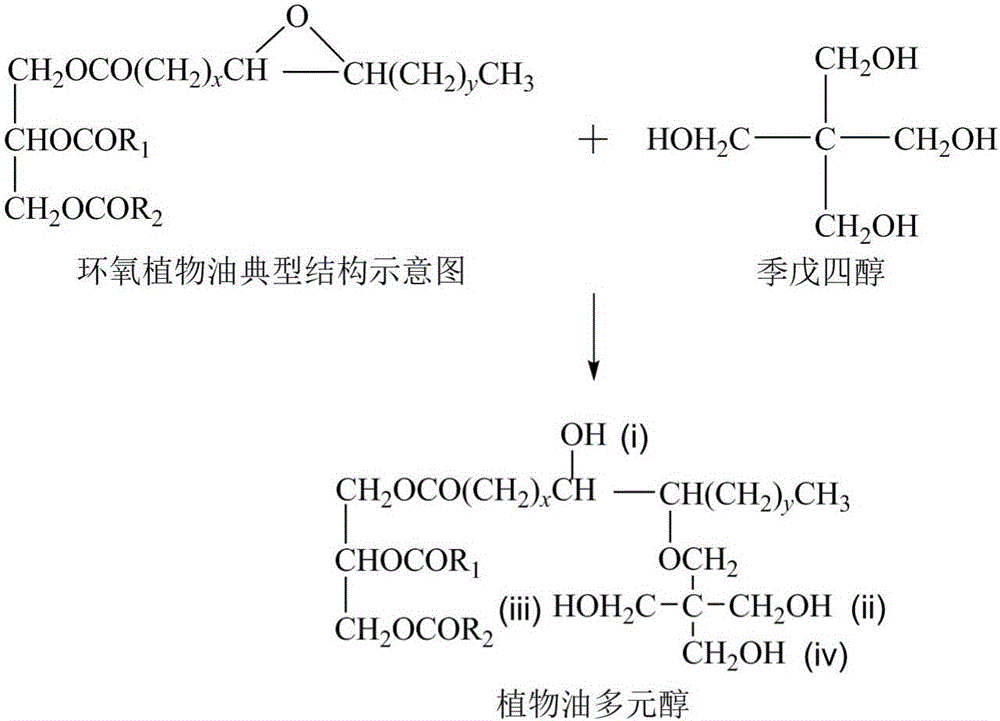
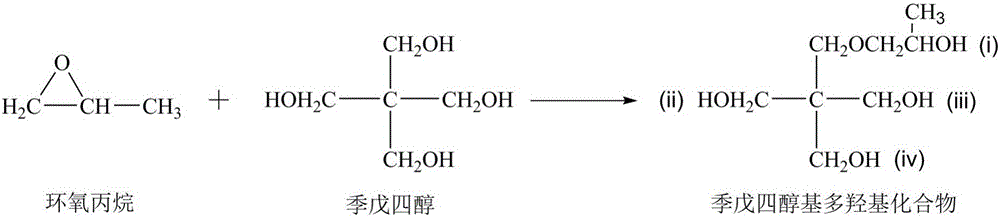
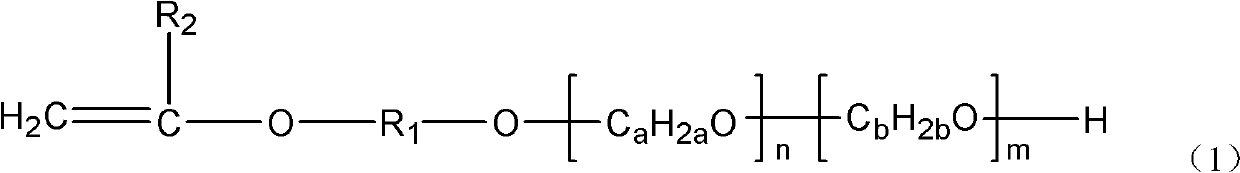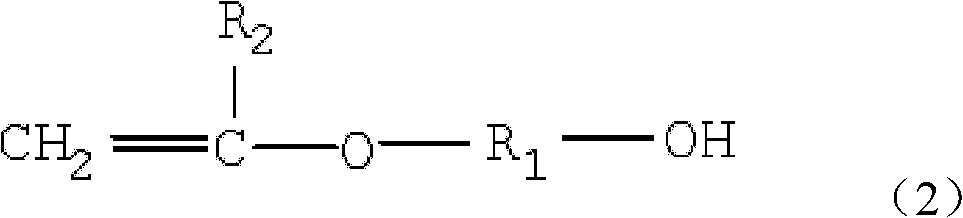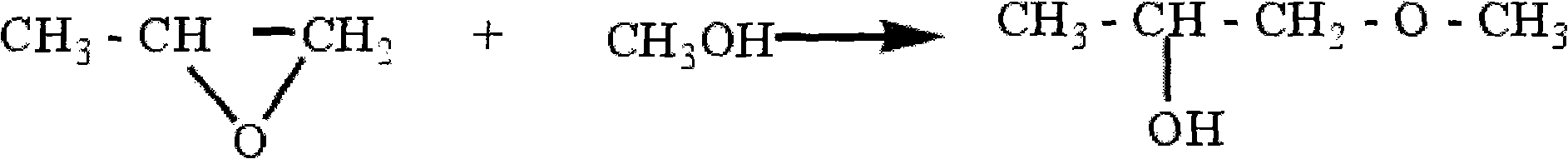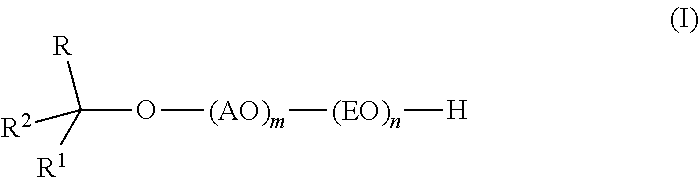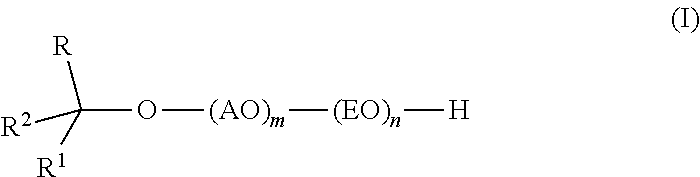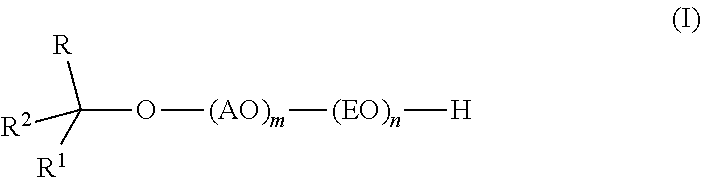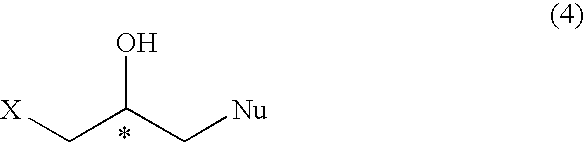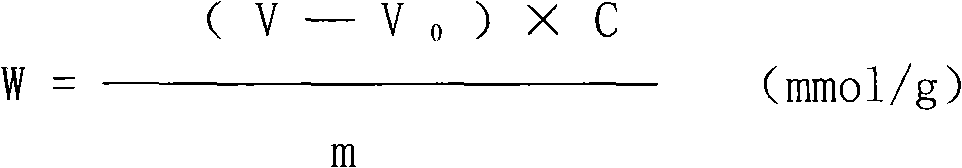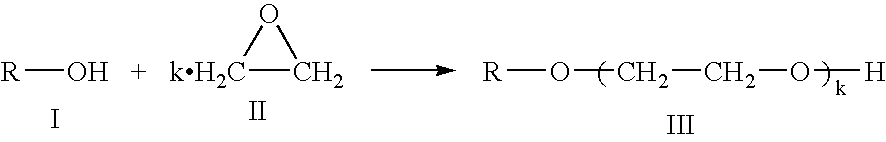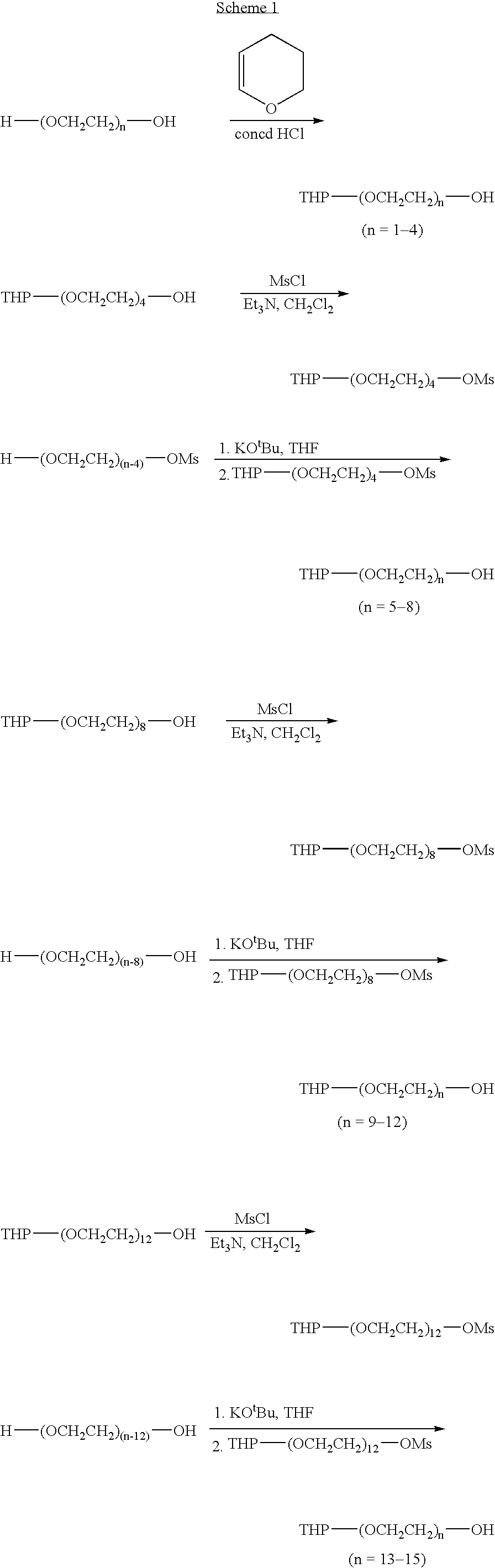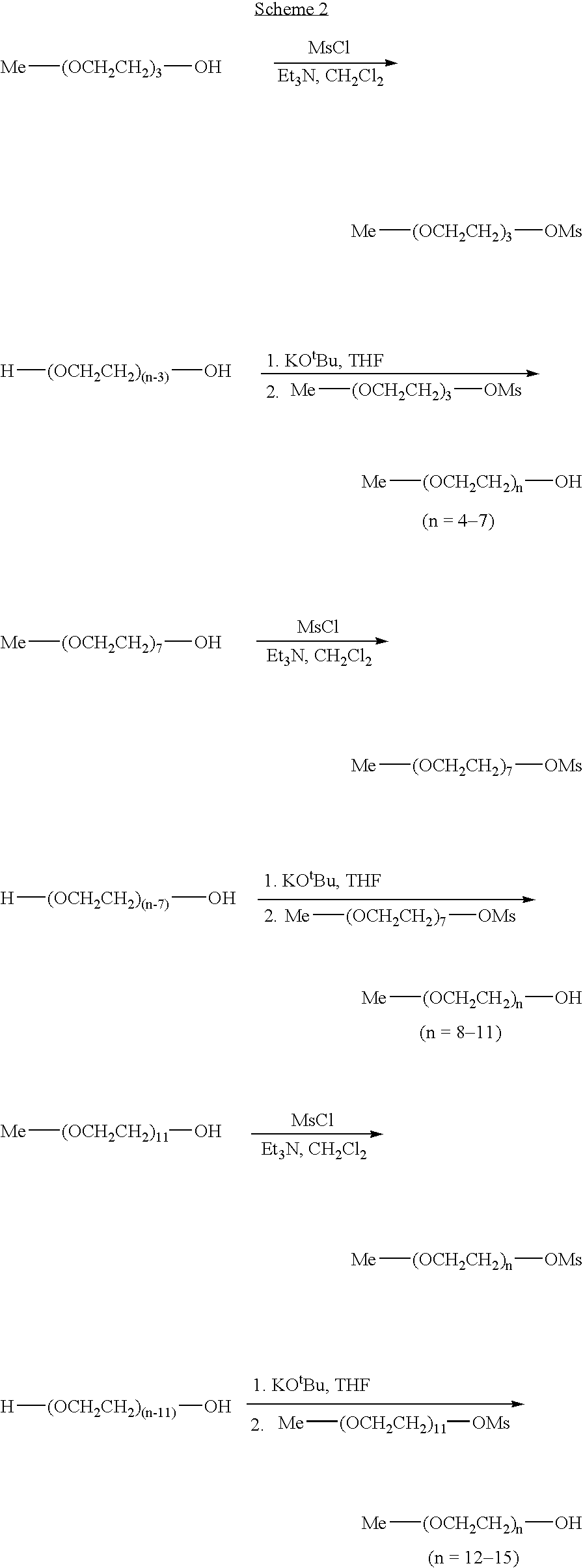Patents
Literature
904results about "Ether preparation from oxiranes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High-stability metal organic skeleton hybrid material, preparation method and application thereof
ActiveCN103694260AHigh hydrothermal stabilitySimple methodOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compoundsMass ratioHybrid material
The invention discloses a high-stability metal organic skeleton hybrid material. The metal organic skeleton hybrid material has high hydrothermal stability, and comprises metal organic skeleton and attapulgite, wherein the mass ratio of the attapulgite to the metal organic skeleton is (0.005-0.7):1.
Owner:NANJING TECH UNIV
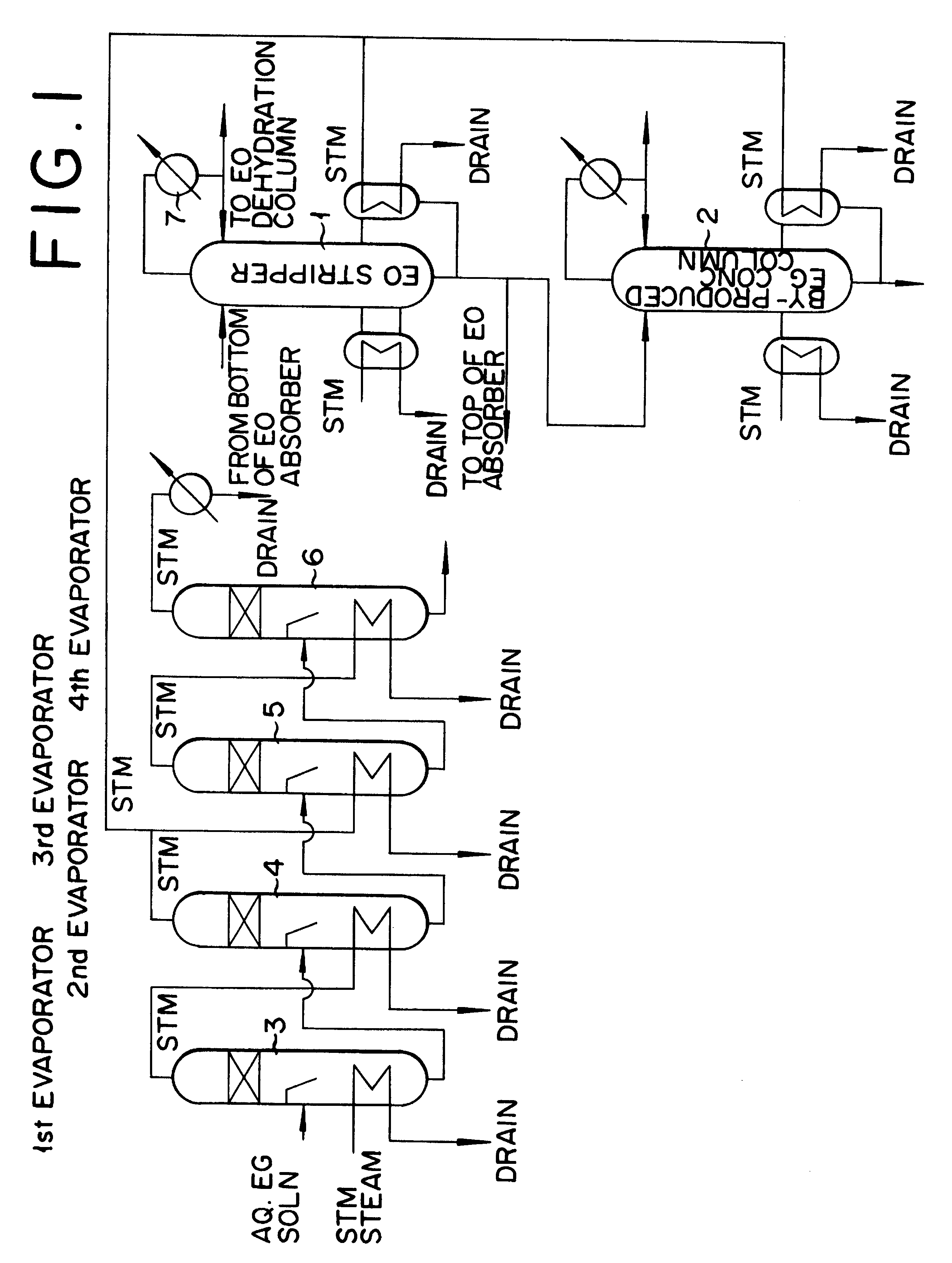
Method for production of ethylene oxide
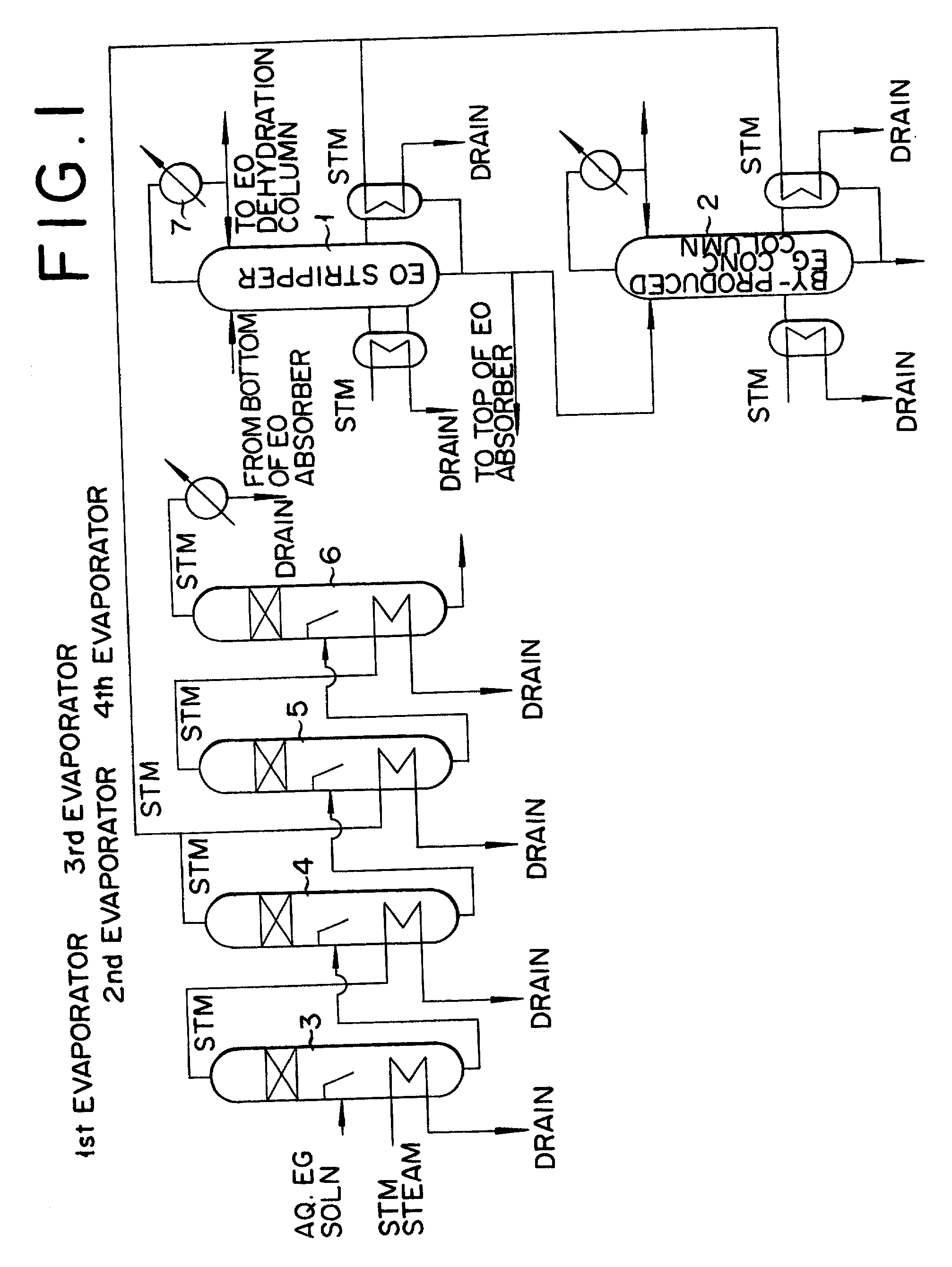
InactiveUS20020010378A1Efficient comprehensive utilizationEffective utilization can be attainedOxygen-containing compound preparationOrganic compound preparationEthylene oxideMultiple-effect evaporator
In a composite process for subjecting ethylene to catalytic gas phase oxidation thereby obtaining ethylene oxide and causing this ethylene oxide to react with water thereby obtaining ethylene glycol, a method for producing the ethylene glycol is provided which permits effective utilization of the energy at the step for dehydrating and concentrating the resultant aqueous ethylene glycol solution. In the production of ethylene glycol by the supply of the aqueous ethylene glycol solution to a concentrating treatment at the multi-effect evaporator, the method contemplated by this invention for the production of ethylene glycol comprises utilizing as the source of heating at least one specific step the steam generated in the multi-effect evaporator.
Owner:RECOVERYCARE COM +1
Process for the start-up of an epoxidation process, a process for the production of ethylene oxide, a 1,2-diol, a 1,2-diol ether, a 1,2-carbonate, or an alkanolamine
InactiveUS20090281339A1Improve catalytic selectivityHigh selectivityProductsOrganic compound preparationOrganic chloride compoundOxygen
A process is provided for the start-up of an ethylene epoxidation process comprising: (a) contacting a catalyst bed comprising a high selectivity epoxidation catalyst with a feed comprising ethylene, oxygen and an organic chloride for a period of time until an increase of at least 1×10−5 mole-% of vinyl chloride (calculated as the moles of vinyl chloride relative to the total gas mixture), preferably 2×10−5 mole-% of vinyl chloride is detected in a reactor outlet gas or a recycle gas loop; and (b) subsequently adjusting the quantity of organic chloride in the feed to a value sufficient to produce ethylene oxide at a substantially optimum selectivity.
Owner:SHELL OIL CO
Vinyl polyether and preparation method and application thereof
InactiveCN102146159AMolecular weight controllableNarrow molecular weight distributionTransportation and packagingMixingVinyl etherHydrogen
The invention provides vinyl polyether. The structure of the vinyl polyether is expressed in the following formula (1), wherein R1 represents C1 to C8 alkylene; R2 represents hydrogen or C1 to C3 alkyl; a and b are integers in the range of 2 to 4 and are unequal; n is an integer in the range of 0 to 100; m is an integer in the range of 0 to 100; and the sum of n and m is more than or equal to 3 and is less than or equal to 100. A polyether monomer has good polymerization activity and molecular structure controllability. The invention also provides a preparation method of the vinyl polyether and application of the vinyl polyethe to the synthesis of high molecular polymers such as a concrete poly carboxylic acid water reducing agent, a water treatment agent, a macromolecule surfactant or various resins and the like.
Owner:LIAONING OXIRANCHEM INC +2
Method for synthesizing propylene glycol methyl ether
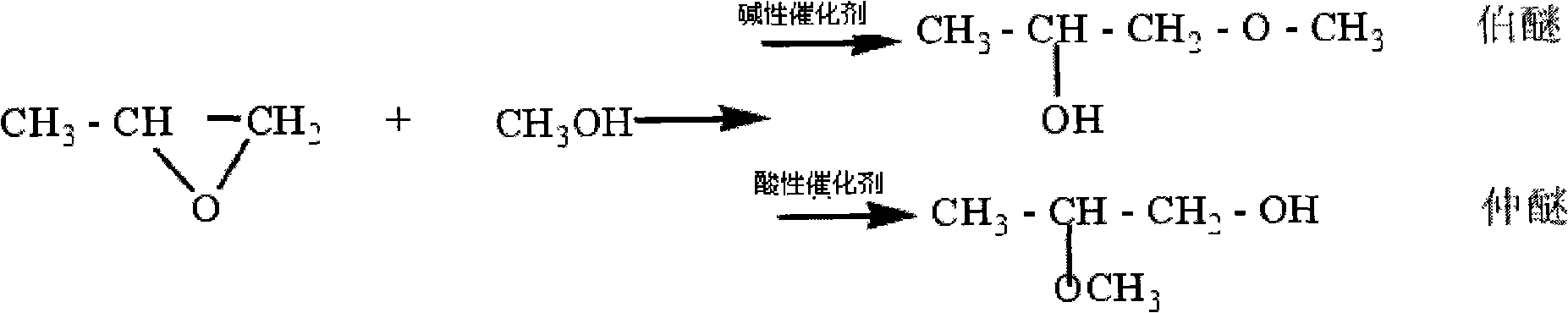
InactiveCN101550069ANo pollutionMild reaction conditionsOrganic-compounds/hydrides/coordination-complexes catalystsEther preparation from oxiranes1-PropanolReaction temperature
A method for synthesizing propylene glycol methyl ether relates to a method for synthesizing propylene glycol methyl ether. The method of the invention settles the problems of high reaction temperature, high pressure, low activity of catalyst and low selectivity existing in the prior method for preparing propylene glycol methyl ether. The method comprises the following steps: 1. preparing a compound A; 2. preparing a compound B; and 3. after naturally cooling the mixed solution B to a room temperature, atmospherically distilling and obtaining the propylene glycol methyl ether. The method of the invention has the advantages of simple process, applicable whole reaction in the condition of normal pressure and low-temperature, 93.3%-94.2% of epoxy propane conversion rate, 98.1%-99.1% of product selectivity, and no environment pollution as the whole reaction process is executed under the condition of no dissolvent. The propylene glycol methyl ether (1-methoxy-2-propanol or 2-methoxy-1-propanol) synthesized according to the invention can be synthesized by regulating the mixed liquid of acetate methyl glyoxaline hydroxide or acetate methyl glyoxaline chloride and ferric trichloride.
Owner:HARBIN NORMAL UNIVERSITY
Cardanol polyoxyethylene ether and preparation method thereof
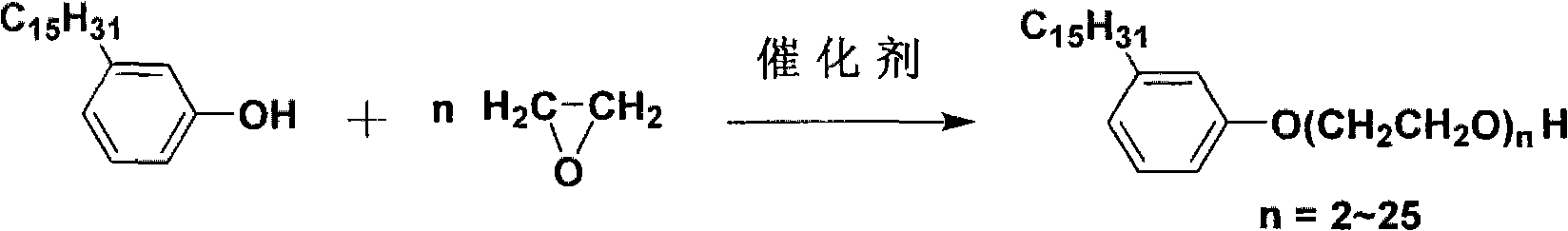
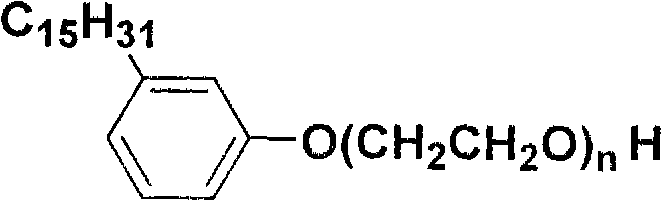
InactiveCN101941894ALow priceReduce adverse effectsEther preparation from oxiranesAcetic acidEthylene oxide
The invention provides cardanol polyoxyethylene ether and a preparation method thereof. The cardanol polyoxyethylene ether is prepared by reacting cardanol and epoxy ethane. The method comprises the following steps of: adding a catalyst and the cardanol into a reaction kettle during a reaction; fully stirring at the temperature of between 150 and 180 DEG C; introducing the epoxy ethane into the reaction kettle; performing polyreaction with stirring; adding acetic acid after the reaction for neutralization; and discharging after cooling so as to obtain the cardanol polyoxyethylene ether. The cardanol polyoxyethylene ether and the preparation method thereof have the advantages of a small number of side reactions, good color and luster of a product, mild reaction condition and low cost of raw materials. Natural cardanol is taken as a raw material for producing, so that the adverse effect of alkylpheol ethoxylates synthesized from petroleum on the environment is avoided. The cardanol polyoxyethylene ether belongs to linear chain alkylpheol ethoxylates and the hydrophile-lipophile balance (HLB) of a nonionic surfactant can be controlled by adjusting the adduct number of the epoxy ethane according to a process, so that the cardanol polyoxyethylene ether can be taken as an emulsifier, a lubricant, a washing agent, a solubilizing agent and the like.
Owner:BINZHOU MEIDONG RESIN
Branched secondary alcohol alkoxylate surfactants and process to make them
InactiveUS20110319669A1Low levelSurface-active detergent compositionsEther preparation from oxiranesAlcoholSURFACTANT BLEND
Provided are alkoxylates of the formula I:wherein AO, EO, m, n, R, R1 and R2 are as defined below. Also provided are processes for making alkoxylates of formula I. The processes provide alkoxylates that exhibit narrow molecular weight distribution and low amounts of residual unreacted alcohol. The alkoxylates have utility in a variety of applications, such as use as surfactants.
Owner:DOW GLOBAL TECH LLC
Process for synthesizing propylene glycol ether
ActiveCN1944365AEasy to separateEliminate the purification processEther preparation from oxiranesEpoxyAlcohol
The present invention discloses process of synthesizing propylene glycol ether. Alcohol, propylene and hydrogen peroxide in the molar ratio of (1-60) to (0.5-10) to 1 are set inside a reactor with catalyst, propylene and hydrogen peroxide produce epoxidation reaction to produce epoxy propane, and the produced epoxy propane and alcohol produce etherification reaction to produce propylene glycol ether. The reaction conditions include reaction temperature of 0-200 deg.c, reaction pressure of 0.5-8.5 MPa, and space velocity of liquid hydrogen peroxide and alcohol of 0.1-65 / hr. The present invention has simple process, environment friendship and propylene glycol ether selectivity up to 90 %.
Owner:HUNAN CHANGLING PETROCHEM SCI & TECH DEV CO LTD +1
Alkoxylation products and process for preparing them by means of DMC catalysts
Novel alkoxylation products containing lateral hydroxyl groups or bearing lateral C—C double bonds and a process for preparing them by means of an alkoxylation reaction of halogenated alkylene oxides using double metal cyanide (DMC) catalysts and subsequent elimination of chlorine.
Owner:EVONIK DEGUSSA GMBH
Quaternized fatty amines, amidoamines and their derivatives from natural oil metathesis
Quaternary ammonium, betaine, or sulfobetaine compositions derived from fatty amines, wherein the fatty amine is made by reducing the amide reaction product of a metathesis-derived C10-C17 monounsaturated acid, octadecene-1,18-dioic acid, or their ester derivatives and a secondary amine, are disclosed. Quaternary ammonium, betaine, or sulfobetaine compositions derived from fatty amidoamines, wherein the amidoamine is made by reacting of a metathesis-derived C10-C17 monounsaturated acid, octadecene-1,18-dioic acid, or their ester derivatives and an aminoalkyl-substituted tertiary amine, are also disclosed. The quaternized compositions are advantageously sulfonated or sulfated. In one aspect, the ester derivative of the C10-C17 monoun-saturated acid or octadecene-1,18-dioic acid is a lower alkyl ester. In other aspects, the ester derivative is a modified triglyceride made by self-metathesis of a natural oil or an unsaturated triglyceride made by cross-metathesis of a natural oil with an olefin. The quaternary ammonium, betaine, and sulfobetaine compositions and their sulfonated or sulfated derivatives are valuable for a wide variety of end uses, including cleaners, fabric treatment, hair conditioning, personal care (liquid cleansing products, conditioning bars, oral care products), antimicrobial compositions, agricultural uses, and oil field applications.
Owner:STEPAN COMPANY
Composite method of isopentenol polyoxyethylene ether
InactiveCN101928392AStrong dispersion retention abilitySimple processEther preparation from oxiranesPolymer scienceEthylene oxide
The invention discloses a preparation method of isopentenol polyoxyethylene ether. Isopentenol and ethylene oxide are served as raw materials; in the presence of catalyst, the raw materials react for 5-40 hours to obtain the isopentenol polyoxyethylene ether at the reaction temperature of 70-160 DEG C. The isopentenol polyoxyethylene ether has the molecular weight of 200-6000 and the iodine value of 3.9-123; the mass ratio of isopentenol to ethylene oxide in the raw materials is 1:0.0143-0.754; and the mass of the catalyst is 0.03-0.5% of total mass of the raw materials of isopentenol and ethylene oxide. The invention has simple and reasonable technology. Because the invention has the structural particularity of branched chain methyl, molecular space steric hindrance is increased, and the composite polycarboxylic acid high-efficiency water reducer has strong cement particle dispersibility holding capacity. Thus, the product has the advantages of low dosage, high water-reducing ratio, good strengthening effects, good durability and the like, does not erode reinforcing steel bars and protects environment.
Owner:ZHEJIANG HUANGMA TECH
Catalyst, a process for preparing the catalyst and a process for the production of an olefin oxide, a 1,2-diol, a 1,2 diol ether, or an alkanolamine
ActiveUS20090131695A1High selectivityImprove stabilityOrganic compound preparationHydroxy compound preparationParticulatesAlkaline earth metal
A catalyst which comprises a carrier and silver deposited on the carrier, which carrier has a surface area of at least 1.3 m2 / g, a median pore diameter of more than 0.8 μm, and a pore size distribution wherein at least 80% of the total pore volume is contained in pores with diameters in the range of from 0.1 to 10 μm and at least 80% of the pore volume contained in the pores with diameters in the range of from 0.1 to 10 μm is contained in pores with diameters in the range of from 0.3 to 10 μm; process for the preparation of a catalyst which process comprises depositing silver on a carrier, wherein the carrier has been obtained by a method which comprises forming a mixture comprising: a) from 50 to 95 weight percent of a first particulate a-alumina having a median particle size (d50) of from 5 to 100 μm; b) from 5 to 50 weight percent of a second particulate a-alumina having a d50 which is less than the d50 of the first particulate a-alumina and which is in the range of from 1 to 10 μm; and c) an alkaline earth metal silicate bond material; weight percent being based on the total weight of a-alumina in the mixture; and firing the mixture to form the carrier; a process for the epoxidation of an olefin, which process comprises reacting a feed comprising an olefin and oxygen in the presence of a said catalyst; and a process for preparing a 1,2-diol, a 1,2-diol ether or an alkanolamine.
Owner:SHELL USA INC
Process for preparation of polytrimethylene ether glycols
Owner:EI DU PONT DE NEMOURS & CO
Process for preparation of optically active halogeno hydroxypropyl compound and glycidyl compound
A process for preparing regioselectively an optically active 1-halogeno-2-hydroxypropyl compound of the following formula; wherein X is halogen atom and Nu is a heteroatom having a substituent,and an optically active glycidyl compound of the formula; which comprises reacting an optically active epihalohydrin of the formula; with a neucleophilic agent,in the presence of a metal complex of the formula; wherein n is an integer of 0, 1 or 2, Y1, Y2 and Y3 are hydrogen atom, etc., and Y2 and Y3 may form a ring such as benzene, A is a counterion and M is a metal ion, and further subjecting the compound (4) to reaction with a base to prepare the optically active glycidyl compound (5).
Owner:DAISO CO LTD
Process for preparing a catalyst, the catalyst, and a use of the catalyst
InactiveUS20050222442A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDopantPolymer science
Owner:SHELL OIL CO
Process for preparation of polytrimethylene ether glocols
There is disclosed a process for preparing polytrimethylene ether glycol by acid catalyzed polycondensation, neutralization and contact with filter aid. The process avoids hydrolysis and yet provides product substantially free of catalyst derived end groups.
Owner:EI DU PONT DE NEMOURS & CO
Alkylene oxide-capped secondary alcohol alkoxylates useful as surfactants
Owner:DOW GLOBAL TECH LLC
Method for production of ethylene glycol
InactiveUS6417411B2Efficient comprehensive utilizationSmall consumptionOxygen-containing compound preparationOrganic compound preparationGas phaseEthylene oxide
In a composite process for subjecting ethylene to catalytic gas phase oxidation thereby obtaining ethylene oxide and causing this ethylene oxide to react with water thereby obtaining ethylene glycol, a method for producing the ethylene glycol is provided which permits effective utilization of the energy at the step for dehydrating and concentrating the resultant aqueous ethylene glycol solution. In the production of ethylene glycol by the supply of the aqueous ethylene glycol solution to a concentrating treatment at the multi-effect evaporator, the method contemplated by this invention for the production of ethylene glycol comprises utilizing as the source of heating at least one specific step the steam generated in the multi-effect evaporator.
Owner:RECOVERYCARE COM +1
Method for preparing metal cyanide catalyst complexes using partially miscible complexing agents
InactiveUS20050065383A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPolyolCyanide
Double metal cyanide catalysts (DMC) are complexed with complexing agents that are miscible in poly(propylene oxide) at higher temperatures and immiscible at lower temperatures. The complexing agent is a poly(ethylene oxide) polymer or a copolymer having a poly(ethylene oxide) block. The catalyst is easily removed from a polyether polyol.
Owner:DOW GLOBAL TECH LLC
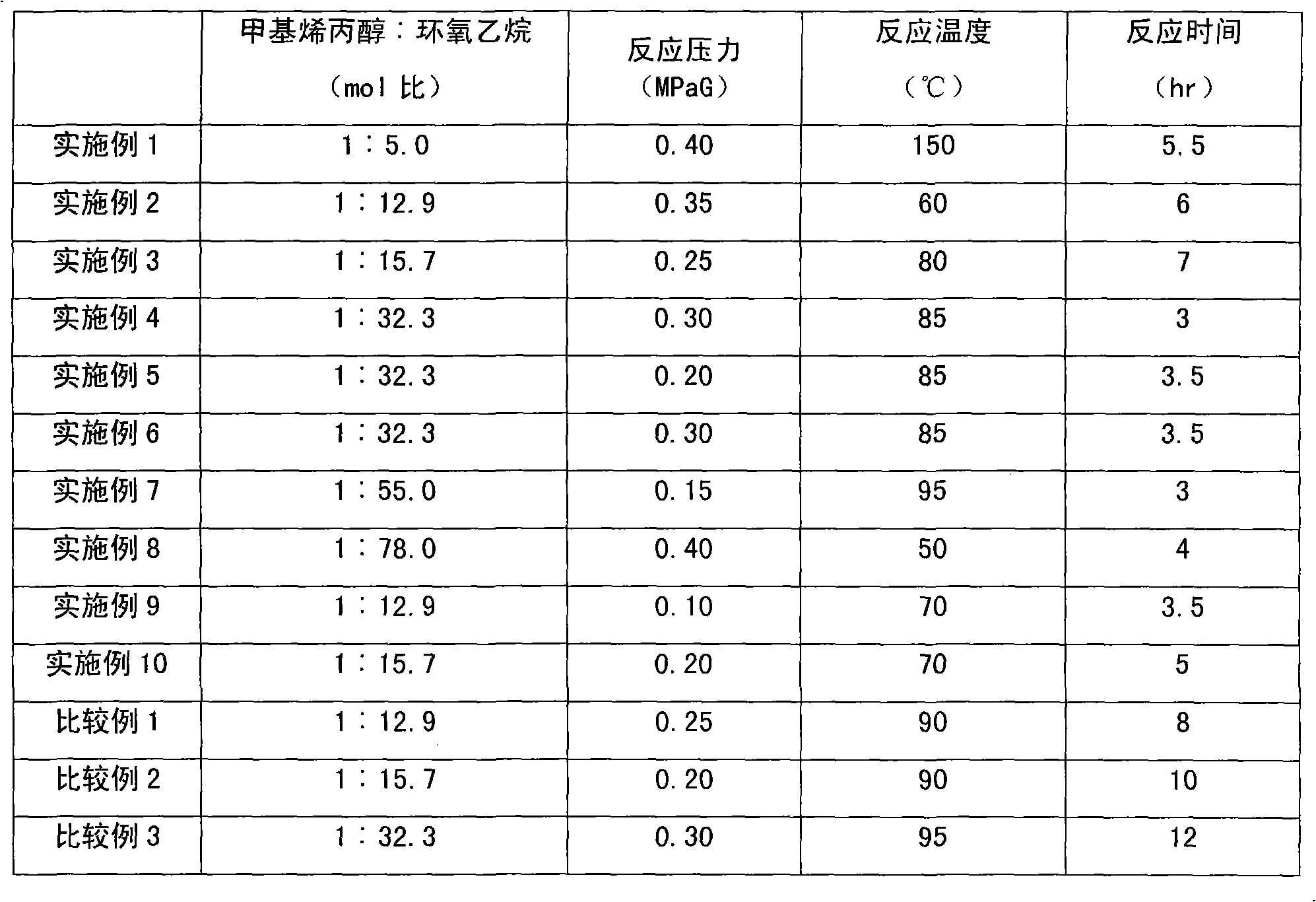
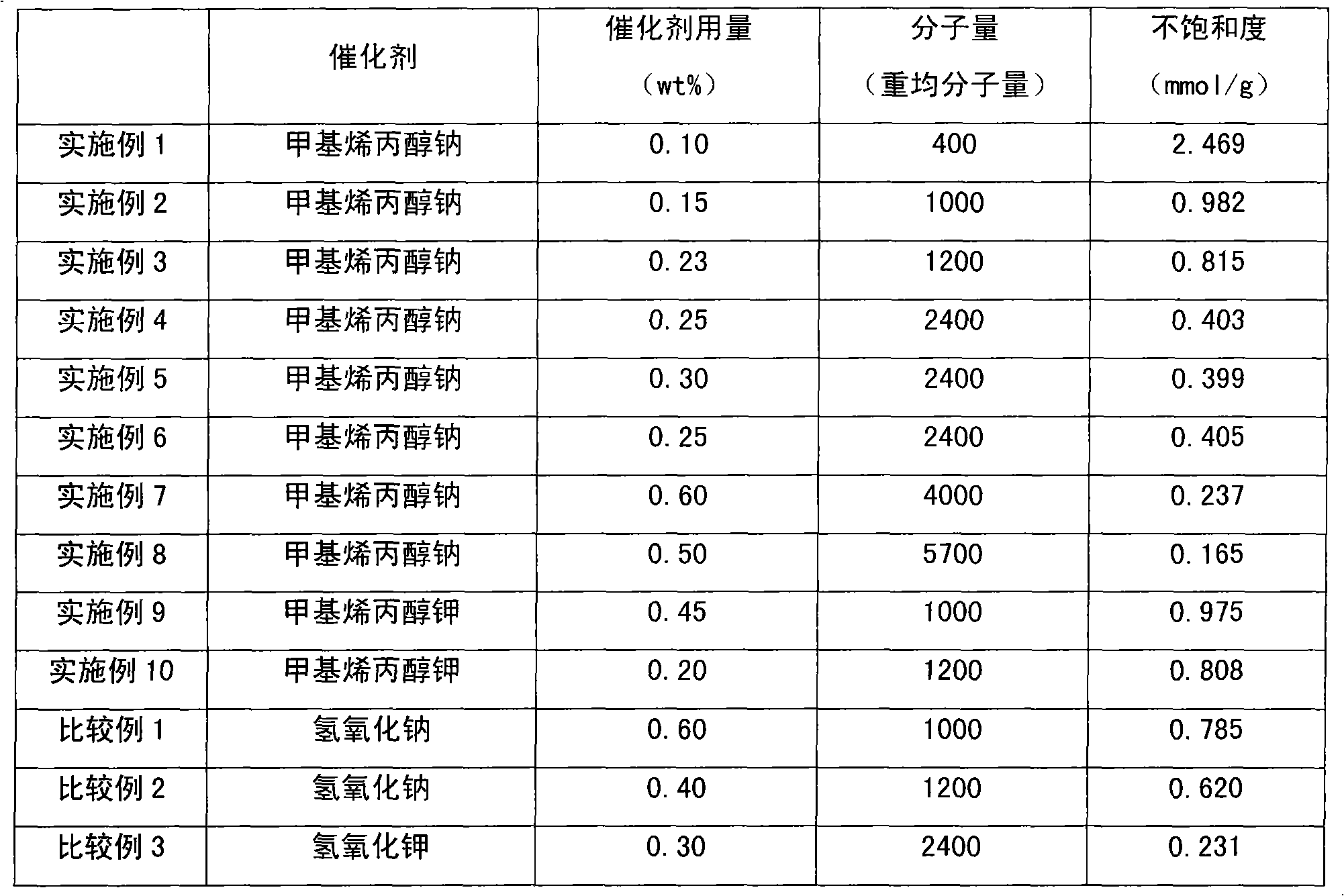
Method for preparing methyl allyl alcohol polyoxyethylene ether
The invention discloses a method for preparing methyl allyl alcohol polyoxyethylene ether. In the method, methallyl alcohol and ethylene oxide undergo a polymerization reaction in the presence of a catalyst to form the methyl allyl alcohol polyoxyethylene ether. The catalyst is sodium methyl allyl alcohol or potassium methyl allyl alcohol; the molar charge ratio of the methallyl alcohol to the ethylene oxide is 1.0:(5.0-78.0); the amount of the catalyst is 0.10 to 0.60 weight percent based on the methallyl alcohol; and the reaction pressure is 0.10 to 0.40MPaG, the reaction temperature is 50 to 150 DEG C, and the reaction time is 3 to 7 hours. The key technical point of the method is that the instauration of the product is increased by over 10 percent by using the more ideal catalyst. The method can be used for preparing methyl allyl alcohol polyoxyethylene ethers with a weight-average molecular weight of 150 to 6,000.
Owner:SHANGHAI DUOLUN CHEM
Method for preparing ethylene glycol phenyl ether, propylene glycol phenyl ether and butylene glycol phenyl ether
InactiveCN101712600AAvoid many problems caused by distillation processLess investmentEther preparation from oxiranesPolymer sciencePhenyl Ethers
The invention discloses a method for preparing ethylene glycol phenyl ether, propylene glycol phenyl ether and butylene glycol phenyl ether, comprising the following steps: 1) firstly, adding phenyl into a reaction kettle, introducing inert gas and adding catalyst, then adding ethylene oxide or propylene oxide or butylene oxide, reacting under the conditions that the temperature is at 70-140 DEG C and the pressure is less than 1 MPa, thereby generating the crude product of ethylene glycol phenyl ether or propylene glycol phenyl ether or butylene glycol phenyl ether, wherein the mol ratio of the added phenyl to the added ethylene oxide or propylene oxide or butylene oxide is 1:0.6-1; and 2) reducing pressure and distilling the prepared crude benzene under the conditions that the vacuum degree is not less than 0.07MPa and the temperature is at 80-110 DEG C, thereby obtaining the product of ethylene glycol phenyl ether or propylene glycol phenyl ether or butylene glycol phenyl ether. The product of ethylene glycol phenyl ether or propylene glycol phenyl ether or butylene glycol phenyl ether prepared by the invention has a purity more than 99%, and does not need to be rectified and purified; and the method has the advantages of low equipment investment, convenient maintenance, simple operation control, low comprehensive cost and the like, and has considerable economic benefit.
Owner:JIAHUA CHEM
Bimaleimide resin toughening modifiers and preparation method thereof
InactiveCN103342892AEliminate the problemImprove performanceOrganic compound preparationEther preparation from oxiranesAlcoholEther
Bimaleimide resin toughening modifiers and a preparation method thereof are disclosed. The preparation method comprises: taking diallyl bisphenol A as the basis, carrying out etherification reaction by using a series of etherification reagents mainly comprising polyhydric alcohols, epoxy compounds, chlorohydrins, choroethers or the like, and reacting 20 parts by mass of diallyl bisphenol A and 15.0-72.0 parts by mass of the ether reagents, under catalysis of 2.2-8.8 parts by mass of an alkali (or an acid), at 60-150 DEG C for 1-10 h, to obtain a series of novel bimaleimide resin modifiers. The modifiers have good solubilizing and toughening effects on BMI bimaleimide resins without inhibition, and accurate control solidification of the bimaleimide resins is easy to realize in a use process.
Owner:XI AN JIAOTONG UNIV
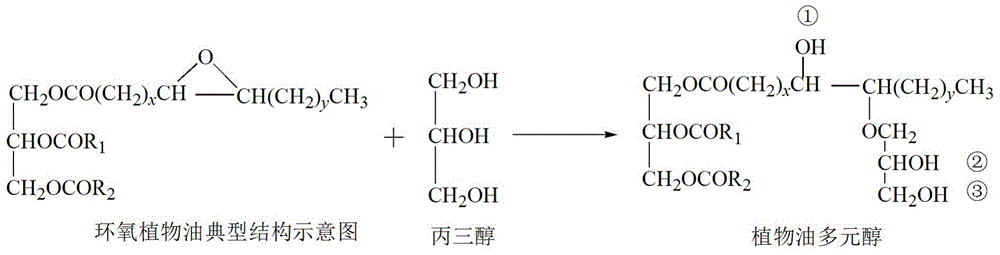
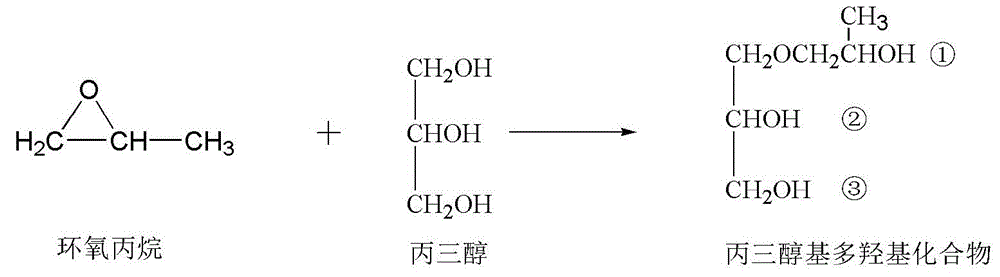
Vegetable oil polyalcohol with high hydroxyl value as well as preparation method and application of vegetable oil polyalcohol
InactiveCN104610060ANovel structureHigh hydroxyl valueOrganic compound preparationPreparation by ester-hydroxy reactionVegetable oilPetrochemical
The invention discloses a vegetable oil polyalcohol with a high hydroxyl value as well as a preparation method and an application of the vegetable oil polyalcohol. The vegetable oil polyalcohol is prepared by carrying out ring-opening reaction and transesterification reaction on epoxy vegetable oil and a polyhydroxy compound. Compared with the prior art, the vegetable oil polyalcohol prepared by adopting a novel ring-opening reagent is novel in structure, high in hydroxyl value, relatively uniform in hydroxyl value distribution, high in primary hydroxyl content, short in suspension chain, low in viscosity and capable of completely substituting the traditional petrochemical polyalcohol to be applied to preparation of a polyurethane foam material. Meanwhile, the preparation method disclosed by the invention is simple in process; the obtained vegetable oil polyalcohol does not need to be after-treated and is very suitable for industrial production; and particularly, after a tubular reactor is selected, the reaction efficiency can be increased, the side reaction can be controlled, and the energy consumption can be reduced.
Owner:NANJING UNIV OF TECH
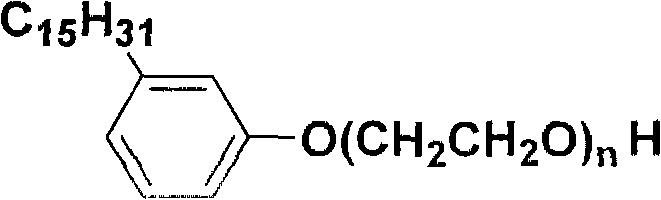
Saturated cardanol polyoxyethylene ether and preparation method thereof
The invention relates to saturated cardanol polyoxyethylene ether and a preparation method thereof, which belongs to the technical field of organic synthesis, and the structural formula of the saturated cardanol polyoxyethylene ether is as follows, wherein n=2-30. The preparation method comprises the following steps: performing a hydrogenation reaction of cardanol; performing filtration and distillation, and performing an addition reaction with ethylene oxide; adjusting the pH value, filtering, and performing reduced-pressure distillation; or performing an addition reaction of cardanol and ethylene oxide; adjusting the pH value, filtering, performing a hydrogenation reaction, filtering, and performing reduced-pressure distillation. The preparation method of the invention uses cardanol as a raw material, and prepares cardanol polyoxyethylene ether with different ethylene oxide adduct numbers and saturated side chains; the product does not contain mixed unsaturated double bond structures, has surface activity, and has stable using performance.
Owner:NORTHEASTERN UNIV
Single reactor synthesis of KOH-capped polyols based on DMC-synthesized intermediates
ActiveUS20050096488A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsElastomerPolyol
The present invention relates to processes for preparing ethylene oxide (EO)-capped polyols in which removal of catalyst residues or salts formed by the neutralization of the basic catalyst is not required prior to discharging the polyol from the reactor because neutralization occurs during or after the starter charge of a subsequent batch. The inventive processes allow for the preparation of DMC-catalyzed intermediates and their base-catalyzed EO caps within the same reactor. Polyols produced by the processes of the invention have a high content of primary hydroxyl groups and may be useful for producing polyurethane foams, elastomers, sealants, coatings, adhesives and the like.
Owner:COVESTRO LLC
Process for preparing an alkoxylated alcohol or phenol
Process for preparing an alkoxylated alcohol comprising reacting a starting monohydroxy alcohol selected from secondary alcohols, tertiary alcohols and mixtures thereof with an alkylene oxide in the presence of hydrogen fluoride and a boron-containing compound comprising at least one B—O bond. The alcohol may also be a primary monohydroxy alcohol when the boron containing compound is boric acid or boric acid anhydride or a mixture thereof, or may be a primary mono hydroxy alcohol, except a C14 / C15 alcohol when reacted with ethylene oxide in the presence of HF and trimethyl borate. A phenol may be alkoxylated in the same way instead of the mono-hydroxyalcohol.
Owner:SHELL OIL CO
Method for degrading and recycling unsaturated polyester resin material
ActiveCN104326907AImprove immersionEfficient fractureOrganic compound preparationPreparation from carboxylic acid anhydridesFiberPolyester
The invention provides a method for degrading and recycling an unsaturated polyester resin material. The method comprises the following steps: preparing a reaction solution by a catalyst and a reaction solvent; mixing the reaction solution with an unsaturated polyester material to prepare an unsaturated polyester degrading system; heating the prepared unsaturated polyester degrading system for degradation; adding a separation solvent into the cooled unsaturated polyester degrading system; filtering to obtain solids, namely enhanced fibers and a catalyst; drying and sieving, and recycling; adding water into filtrate so as to separate out a polymer degrading component containing a styrene structure, and filtering; drying and recycling the filtered polymer solids containing the styrene structure; and evaporating filtrate to obtain substances mainly comprising resin degraded products which do not contain the styrene structure. The method for degrading and recycling the unsaturated polyester resin material has the advantages of low cost, moderate recycling conditions and high degrading activity.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI +1
Vegetable oil polyols with high hydroxyl value as well as preparation method and application of vegetable oil polyols
ActiveCN105669450ANovel structureHigh hydroxyl valuePreparation by ester-hydroxy reactionOrganic compound preparationVegetable oilTransesterification
The invention discloses vegetable oil polyols with high hydroxyl value as well as a preparation method and an application of the vegetable oil polyols. The vegetable oil polyols are prepared from epoxidized vegetable oil and a polyhydroxyl compound through a ring-opening reaction and transesterification. Compared with the prior art, a novel ring-opening reagent is used, the prepared vegetable oil polyols are novel in structure and low in viscosity, has high hydroxyl value and primary hydroxyl content and short pendant chains, are distributed more uniformly and can completely replace traditional petrochemical polyols to be applied to preparation of a polyurethane foam material. Meanwhile, the method adopts simple process, postprocessing is not needed for a product, the method is quite suitable for industrial production, the reaction efficiency can be increased especially after selection of a tubular reactor, generation of side reactions is controlled, and energy consumption is reduced.
Owner:NANJING TECH UNIV
Combinatorial synthesis of PEG oligomer libraries
A simple chain-extending approach was established for the scale-up of the monoprotected monodisperse PEG diol materials. Reactions of THP-(OCH2CH2)n—OMs (n=4, 8, 12) with a large excess of commercially available H—(OCH2CH2)n—OH (n=1-4) under basic conditions led to THP-(OCH2CH2)n—OH (n=5-15). Similarly, Me-(OCH2CH2)n—OH (n=4-11, 13) were prepared from Me-(OCH2CH2)n—OMs (n=3, 7, 11). For the chain elongation steps, 40-80% yields were achieved through extraction purification. PEG oligomer libraries I and II were generated in 50-95% overall yields by alkylation or acylation of THP-(OCH2CH2)n—OH (n=1-15) followed by deprotection. Alkylation of Me-(OCH2CH2)n—OH (n=1-11, 13) with X—(CH2)m—CO2R (X=Br or OMs) and subsequent hydrolysis led to PEG oligomer library III in 30-60% overall yields. Combinatorial purification techniques were adapted to the larger-scale library synthesis. A total of 498 compounds, each with a weight of 2-5 g and a minimum purity of 90%, were synthesized.
Owner:BIOCON LTD
Process for producing polyether polyol
InactiveUS20080071118A1Less coloringOrganic compound preparationEther preparation from oxiranesPolyolDesorption
To provide a method of producing a polyether polyol having less coloration with good selectivity and high efficiency by dehydrocondensing a polyol. In producing a polyether polyol by dehydrocondensation reaction of a polyol, a solid acid catalyst satisfying at least one of the following requirements (1) to (3) is used: (1) Acid function H0 measured by Hammett's indicator adsorption method is larger than −3; (2) In Temperature-Programmed Desorption (TPD) analysis of ammonia, desorption amount of ammonia in a region of from 100 to 350° C. is 60% or more of the entire ammonia desorption amount (a region of from 25 to 700° C.); and (3) In thermogravimetry (TG), desorption amount of water is 3% by weight or more of a reference weight in a region of from 32 to 250° C.
Owner:MITSUBISHI CHEM CORP
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com