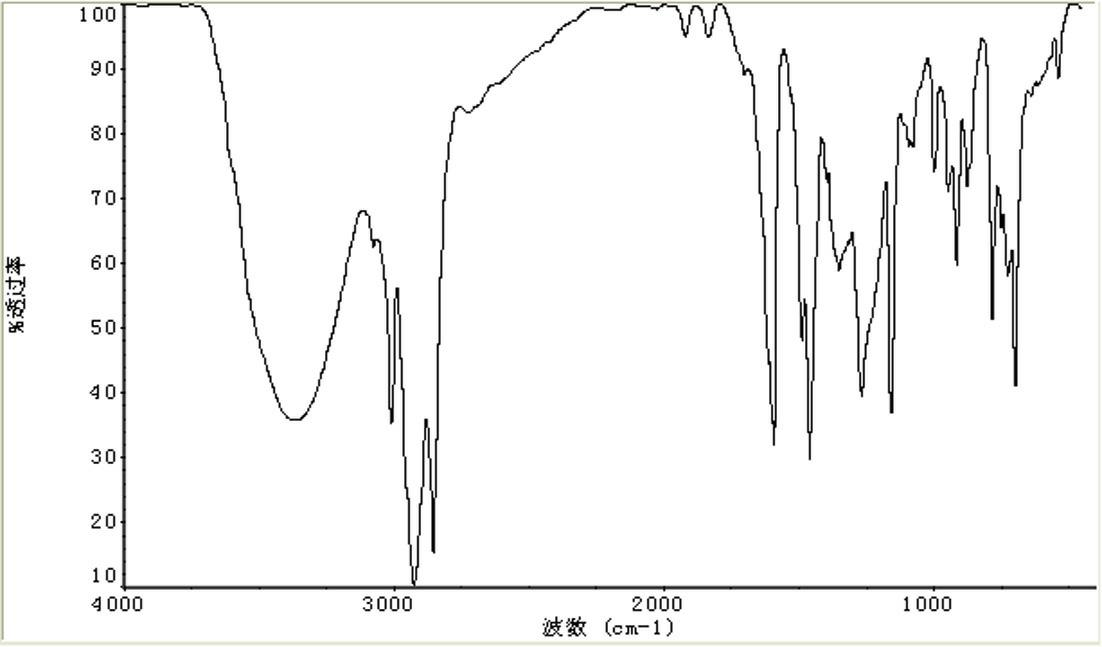
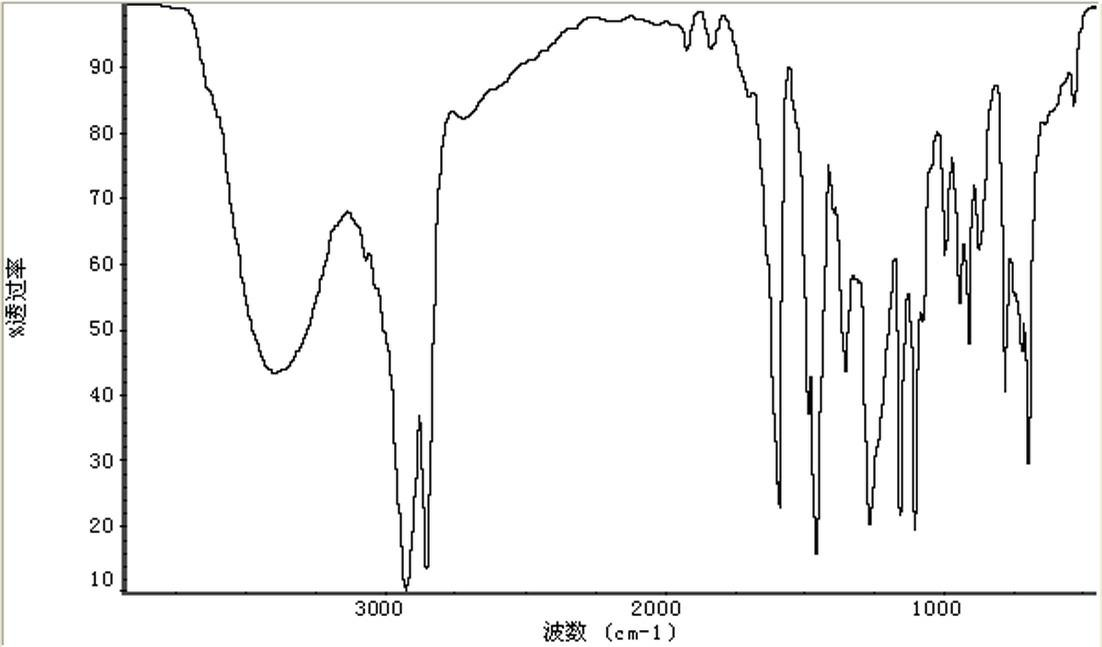
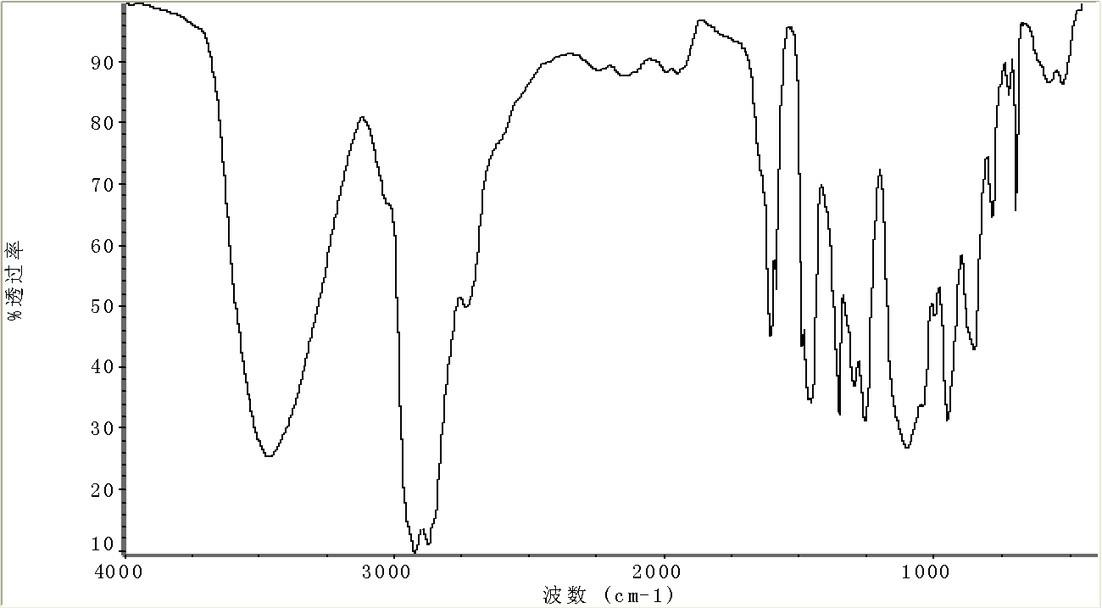
Saturated cardanol polyoxyethylene ether and preparation method thereof
A cardanol polyoxyethylene ether and cardanol technology are applied in the field of saturated cardanol polyoxyethylene ether and its preparation, and achieve the effects of simple process and stable use performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The hydrogenation catalyst used is RTH3110Raney nickel; the organic solvent used is absolute ethanol, the addition catalyst used is NaOH, and the inert gas used is nitrogen;
[0072] Put 720g of cardanol in a high-pressure airtight container, add RTH3110Raney nickel and absolute ethanol, the addition of hydrogenation catalyst is 1 times of the total weight of cardanol; the addition of absolute ethanol is 5 times of the total weight of cardanol; Nitrogen gas was passed into the high-pressure airtight container at 2 L / min for 10 minutes, the air was replaced, and then hydrogen was introduced until the pressure in the high-pressure airtight container was 0.15 MPa. Drop again, and the 6.5h reaction is completed, and a reaction solution is obtained;
[0073] The primary reaction solution is filtered to remove the hydrogenation catalyst, and then the filtrate is subjected to atmospheric distillation at 78-80°C to remove absolute ethanol to obtain an intermediate solution;
...
Embodiment 2
[0079] The hydrogenation catalyst used is RTH3124Raney nickel; the organic solvent used is absolute ethanol, and the addition catalyst used is K 2 CO 3 , the inert gas used is argon;
[0080] 720g cardanol is placed in a high-pressure airtight container and added RTH3124Raney nickel and dehydrated alcohol, the addition of hydrogenation catalyst is 10 times of the total weight of cardanol; the addition of dehydrated alcohol is 6 times of the total weight of cardanol; Pass argon gas at 2 L / min into the high-pressure closed container for 10 minutes, replace the air, and then pass hydrogen gas into the high-pressure closed container until the pressure in the high-pressure closed container is 0.13 MPa. Drop again, and the 7h reaction is completed, and a reaction solution is obtained;
[0081] The primary reaction solution is filtered to remove the hydrogenation catalyst, and then the filtrate is subjected to atmospheric distillation at 78-80°C to remove absolute ethanol to obtain...
Embodiment 3
[0087] The hydrogenation catalyst adopted is RTH3146Raney nickel; the organic solvent adopted is tetrahydrofuran, and the addition catalyst adopted is Na 2 CO 3 , the inert gas used is CO 2 ;
[0088] Put 720g of cardanol into a high-pressure airtight container, add RTH3110Raney nickel and tetrahydrofuran, the addition of hydrogenation catalyst is 4 times of the total weight of cardanol; the addition of tetrahydrofuran is 4 times of the total weight of cardanol; Inject CO at 2 L / min 2 10min, replace the air, and then pass in hydrogen until the pressure in the high-pressure airtight container is 6MPa, and then react at room temperature and with a stirring speed of 180r / min until the hydrogen pressure no longer drops, and the reaction is completed in 5.5h, and a reaction solution is obtained ;
[0089] The primary reaction solution is filtered to remove the hydrogenation catalyst, and then the filtrate is subjected to atmospheric distillation at 65~67°C to remove tetrahydrof...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com