Preparation method of reactive gemini quaternary ammonium salt leather bactericide
A technology of gemini quaternary ammonium salt and bactericide is applied in the field of preparation of reactive gemini quaternary ammonium salt leather bactericide, can solve problems such as high toxicity, and achieve the effect of promoting bactericidal effect, easy biodegradation and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
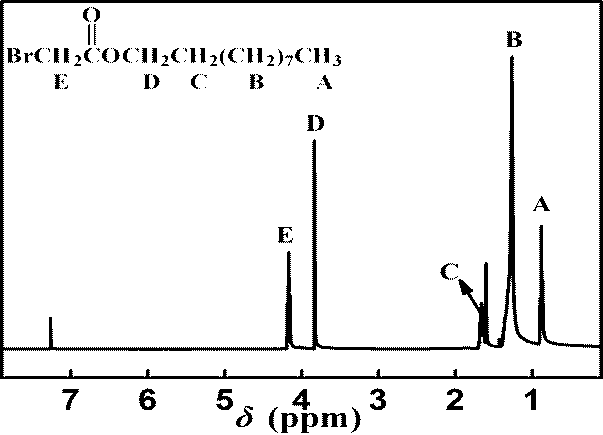
[0028] (1) Preparation of n-octyl bromoacetate:
[0029] Add 4.84 g (24 mmol) of bromoacetyl bromide, 2.61 g (20 mmol) of n-octanol, 0.49 g (4 mmol) of catalyst and 30 mL of dichloromethane into a 250 mL three-neck flask, react at room temperature for 2-3 h, add 20 mL Distilled water to remove excess bromoacetyl bromide, extracted the organic phase with dichloromethane, obtained the crude product by rotary evaporation of the organic phase, and further purified by flash column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain n-octyl bromoacetate (4.37 g), 87% yield.
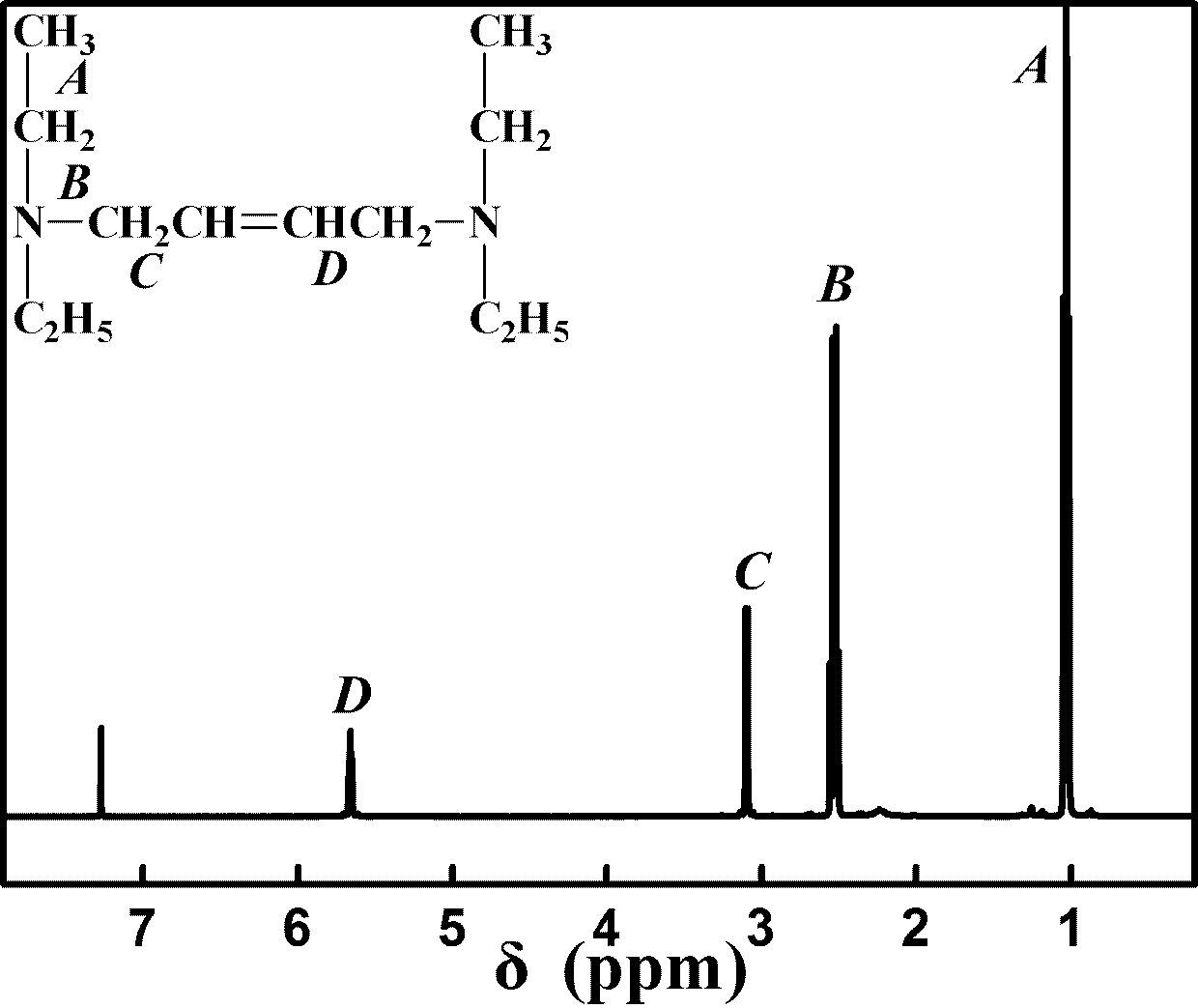
[0030] (2) Preparation of trans-N,N,N',N'-tetraethyl-2-butene-1,4-diamine
[0031]Add 0.19 g (15 mmol) of trans-1,4-dichloro-2-butene, 16.26 g (225 mmol) of diethylamine and 30 mL of solvent into a 250 mL three-necked flask, react at 50~70°C for 3~5 h, filter The precipitate was removed, and the crude product was obtained by rotary evaporation of the filtrate, which was further purified by flash ...
Embodiment 2
[0035] (1) Preparation of n-decyl bromoacetate:
[0036] Add 4.84 g (24 mmol) of bromoacetyl bromide, 3.17 g (20 mmol) of n-decyl alcohol, 0.49 g (4 mmol) of catalyst and 30 mL of dichloromethane into a 250 mL three-necked flask, react at room temperature for 2-3 h, add 20 mL Distilled water to remove excess bromoacetyl bromide, extracted the organic phase with dichloromethane, obtained the crude product by rotary evaporation of the organic phase, and further purified by flash column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain n-decyl bromoacetate (4.96 g), yield 88.8%.
[0037] (2) Preparation of trans-N,N,N',N'-tetraethyl-2-butene-1,4-diamine
[0038] Add 0.19 g (15 mmol) of trans-1,4-dichloro-2-butene, 16.26 g (225 mmol) of diethylamine and 30 mL of solvent into a 250 mL three-necked flask, react at 50~70°C for 3~5 h, The precipitate was removed by filtration, and the crude product was obtained by rotary evaporation of the filtrate, which was further ...
Embodiment 3
[0042] (1) Preparation of n-dodecyl bromoacetate:
[0043] Add 4.84 g (24 mmol) of bromoacetyl bromide, 3.73 g (20 mmol) of n-dodecyl alcohol, 0.49 g (4 mmol) of catalyst and 30 mL of dichloromethane solvent into a 250 mL three-necked flask, and react at room temperature for 2-3 h. Add 20 mL of distilled water to remove excess bromoacetyl bromide, extract the organic phase with dichloromethane, obtain the crude product by rotary evaporation of the organic phase, and further purify by flash column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain bromoacetic acid N-dodecyl ester (5.47 g), yield 89%.
[0044] (2) Preparation of trans-N,N,N',N'-tetraethyl-2-butene-1,4-diamine
[0045] Add 0.19 g (15 mmol) of trans-1,4-dichloro-2-butene, 16.26 g (225 mmol) of diethylamine and 30 mL of solvent into a 250 mL three-necked flask, react at 50~70°C for 3~5 h, filter The precipitate was removed, and the crude product was obtained by rotary evaporation of the filtrate, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com