Method for production of ethylene oxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Now, this invention will be specifically explained below with reference to preferred mode of embodying this invention below.
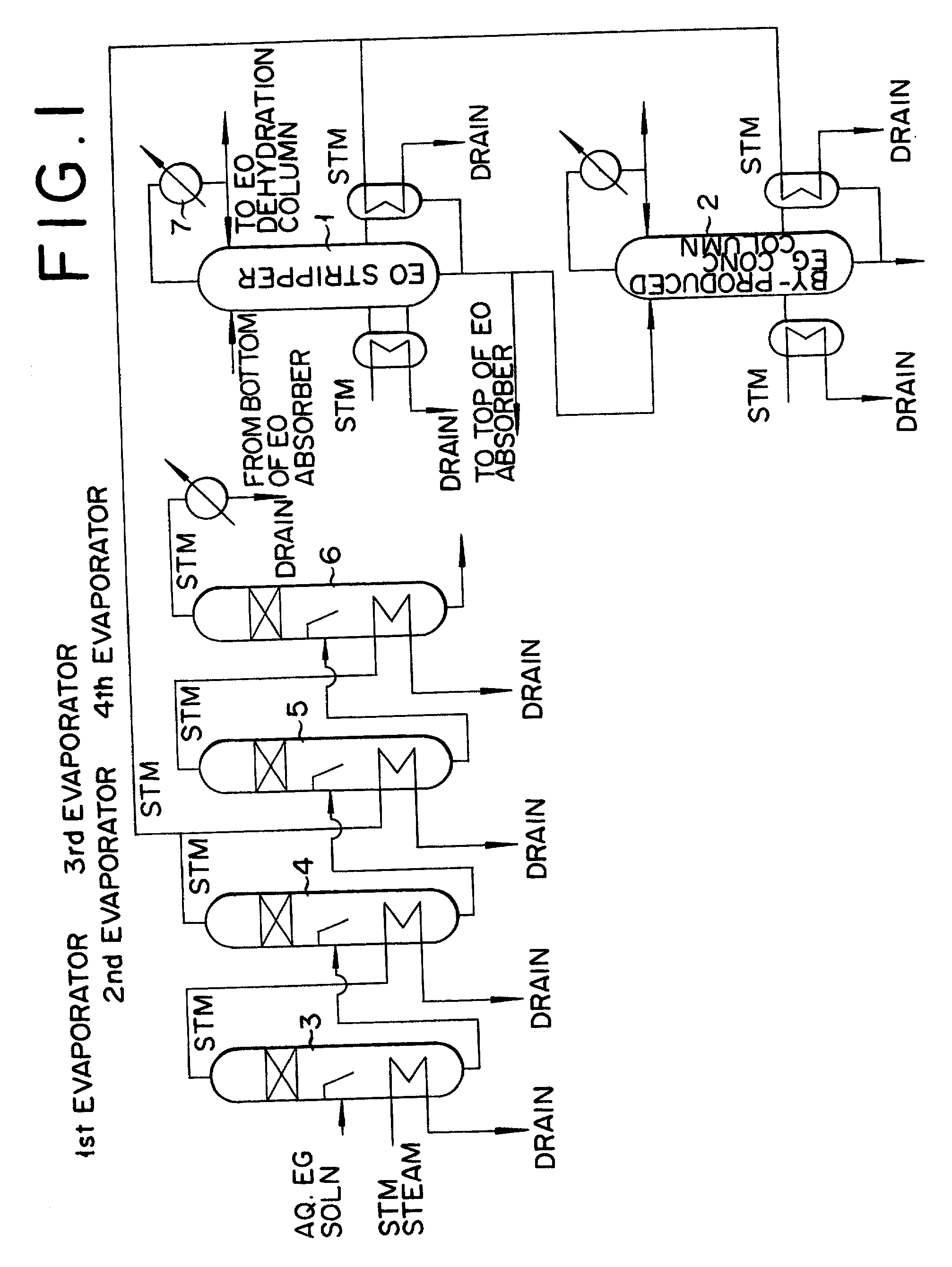
[0025] The reaction of ethylene oxide with water is carried out under the following conditions. A molar ratio of ethylene oxide and water, namely ethylene oxide:water, is in the range of 1:7-1:50, preferably 1:5-1:30. A reaction pressure is in the range of 0.5-3.0 MPa (Gauge), preferably 1.5-2.5 MPa (Gauge), a reaction temperature is in the range of 120.degree.-250.degree. C., preferably 130.degree.-180.degree. C., and a concentration of the formed ethylene glycol was in the range of 5-40 mass %. The reaction is carried out in the batch-wise, the semibatch-wise, or the continuously, which ever fits the occasion best. The ethylene glycol thus obtained is supplied to the multi-effect evaporator and concentrated and dehydrated till a concentration of 40-95 mass % or over.
[0026] The number of multi-effect evaporators is described in detail at pages 428-431 o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com