Cardanol polyoxyethylene ether and preparation method thereof
A technology of cardanol polyoxyethylene ether and cardanol, which is applied in the field of organic compounds and its preparation, can solve the problems of long residual time and environmental pollution, and achieve the effects of low price, good product color and good industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
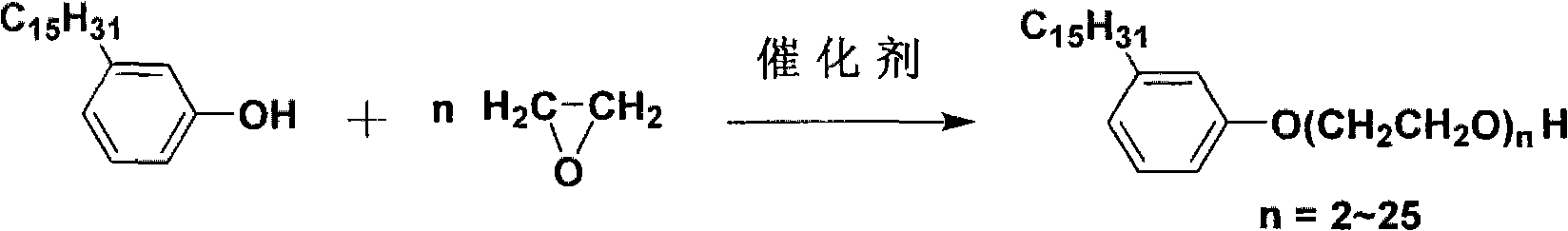
[0009] In the present invention, cardanol is high-purity cardanol extracted from natural cashew nut shells. This cardanol is non-toxic and light in color. With this excellent renewable biomass cardanol, it can be obtained through ethoxylation reaction. Cardanol polyoxyethylene ether with excellent performance, its mechanism is: cardanol produces oxygen negative ions under alkaline conditions, and then ethoxylates with ethylene oxide to obtain cardanol polyoxyethylene ether.
[0010] Its reaction equation is as follows:
[0011]
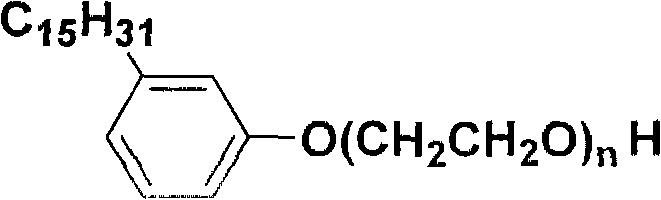
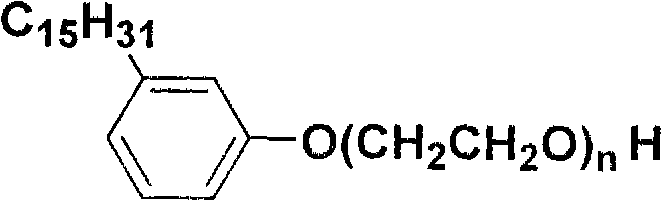
[0012] A cardanol polyoxyethylene ether, which has the following structural formula:
[0013]
[0014] Wherein, n=2-25.
[0015] The preparation method of cardanol polyoxyethylene ether uses cardanol and ethylene oxide to react to prepare cardanol polyoxyethylene ether. During the reaction, the catalyst and cardanol are added to the reaction kettle, the feeding port cover is tightened, and the stirring and heating system is turned on. Simultan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com