New crystalline form of hydrochloric acid Ivabradine and preparation method thereof
A technology of ivabradine hydrochloride and ivabradine, which is applied in the field of new crystal forms of ivabradine hydrochloride and its preparation, and can solve problems not described in detail
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: Ivabradine hydrochloride in W-crystalline form
[0015] 1.0 g of ivabradine hydrochloride was added to 30 mL of acetone and 2 mL of methanol for dissolution under reflux, and the filtrate was allowed to stand for crystallization. Then pump and filter, and wash the filter cake with a small amount of acetone. The filter cake was vacuum dried at 85°C to constant weight. A 0.85 g sample of crystal form W was obtained. The melting point is 196.5-197.7°C.
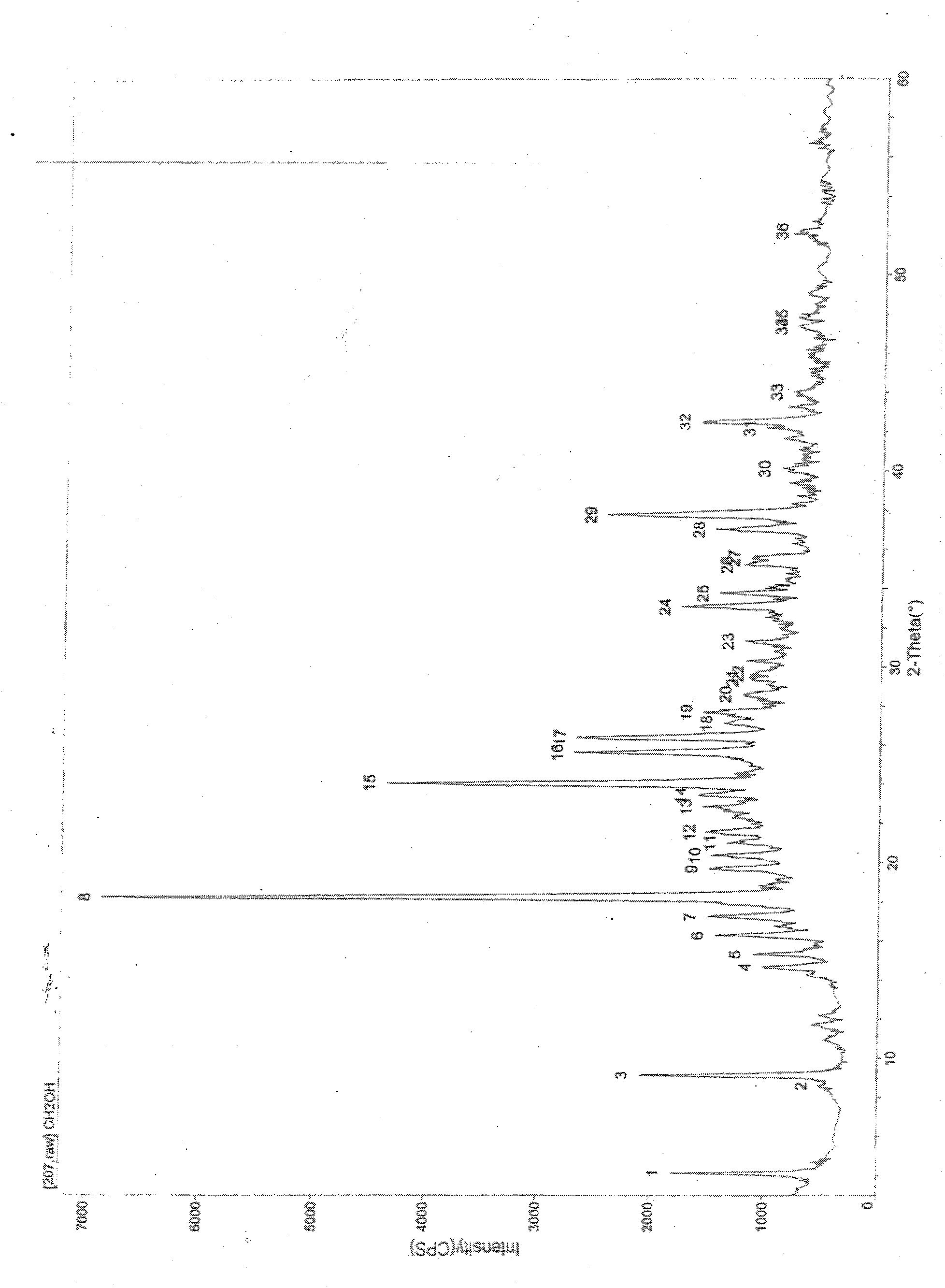
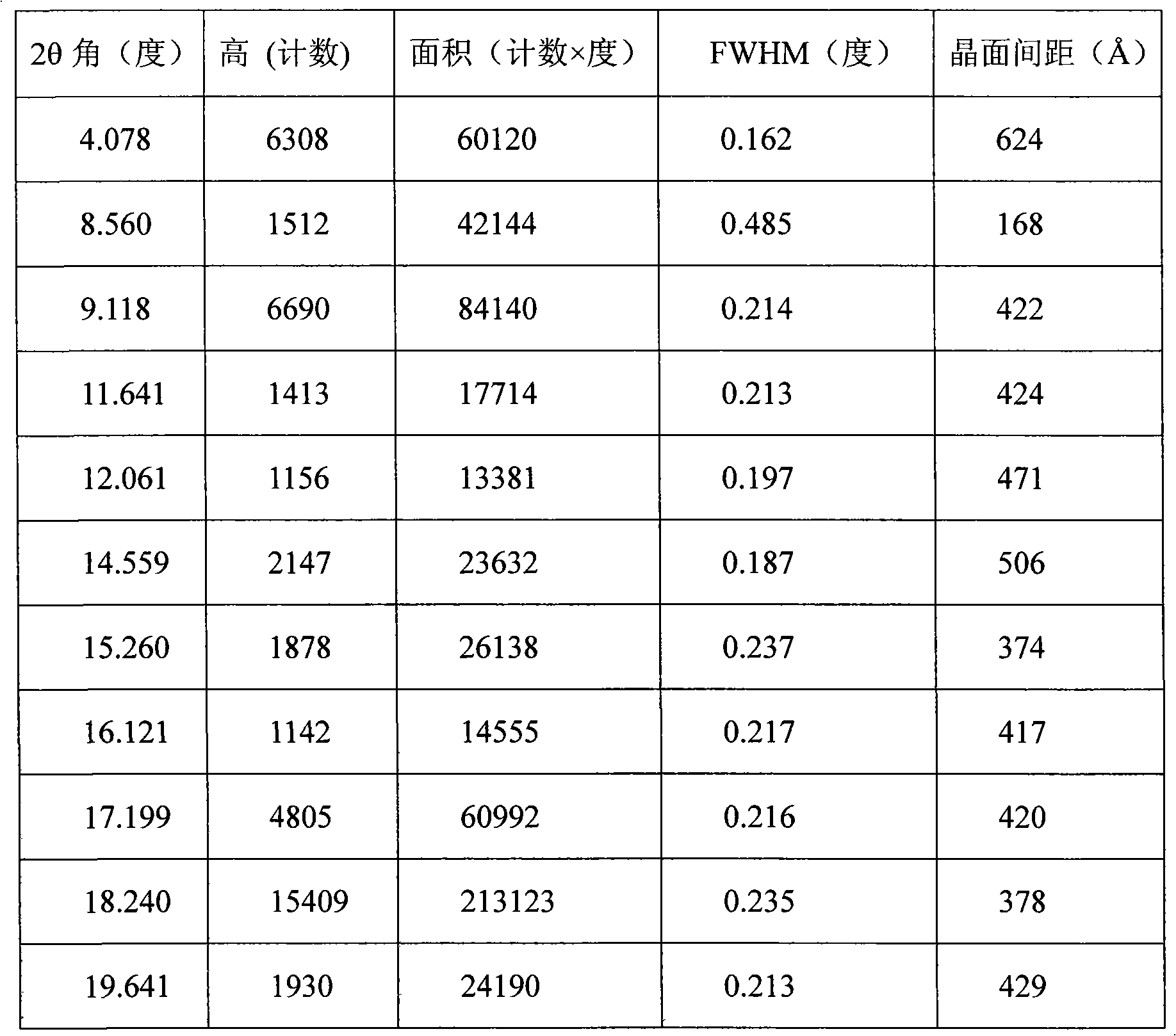
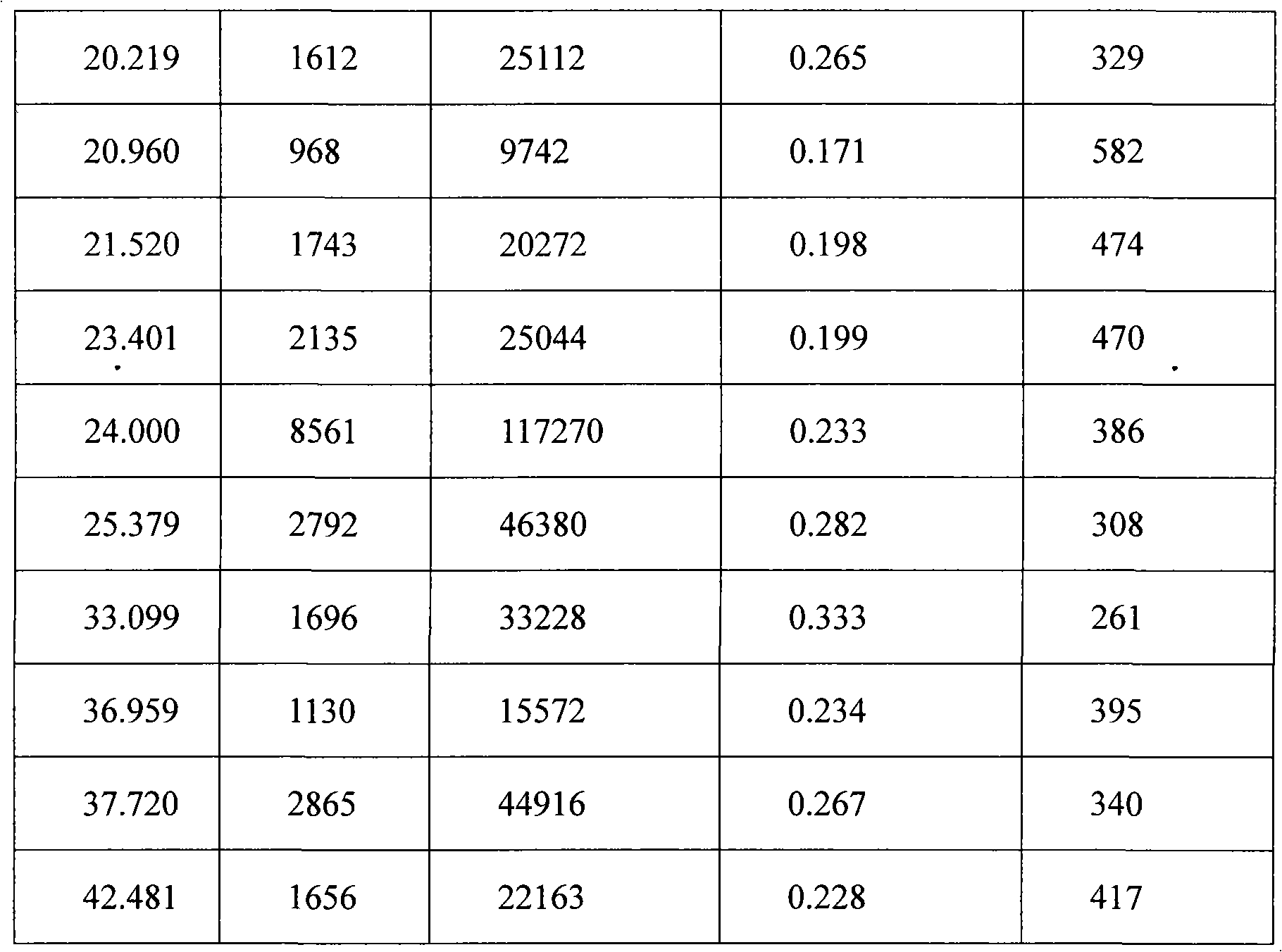
[0016] The powder X-ray diffraction pattern is shown in Figure (1).
[0017]
[0018]
Embodiment 2
[0019] Embodiment 2: pharmaceutical composition
[0020] For the preparation of 1000 tablets, wherein each tablet contains 5 mg of ivabradine base, using the following formula 5.39 g of the compound of Example 1, 60.0 g of lactose, 20.0 g of microcrystalline cellulose, and 1.0 mg of magnesium stearate g, 13.61 g of starch, prepared by a conventional tablet preparation process.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com