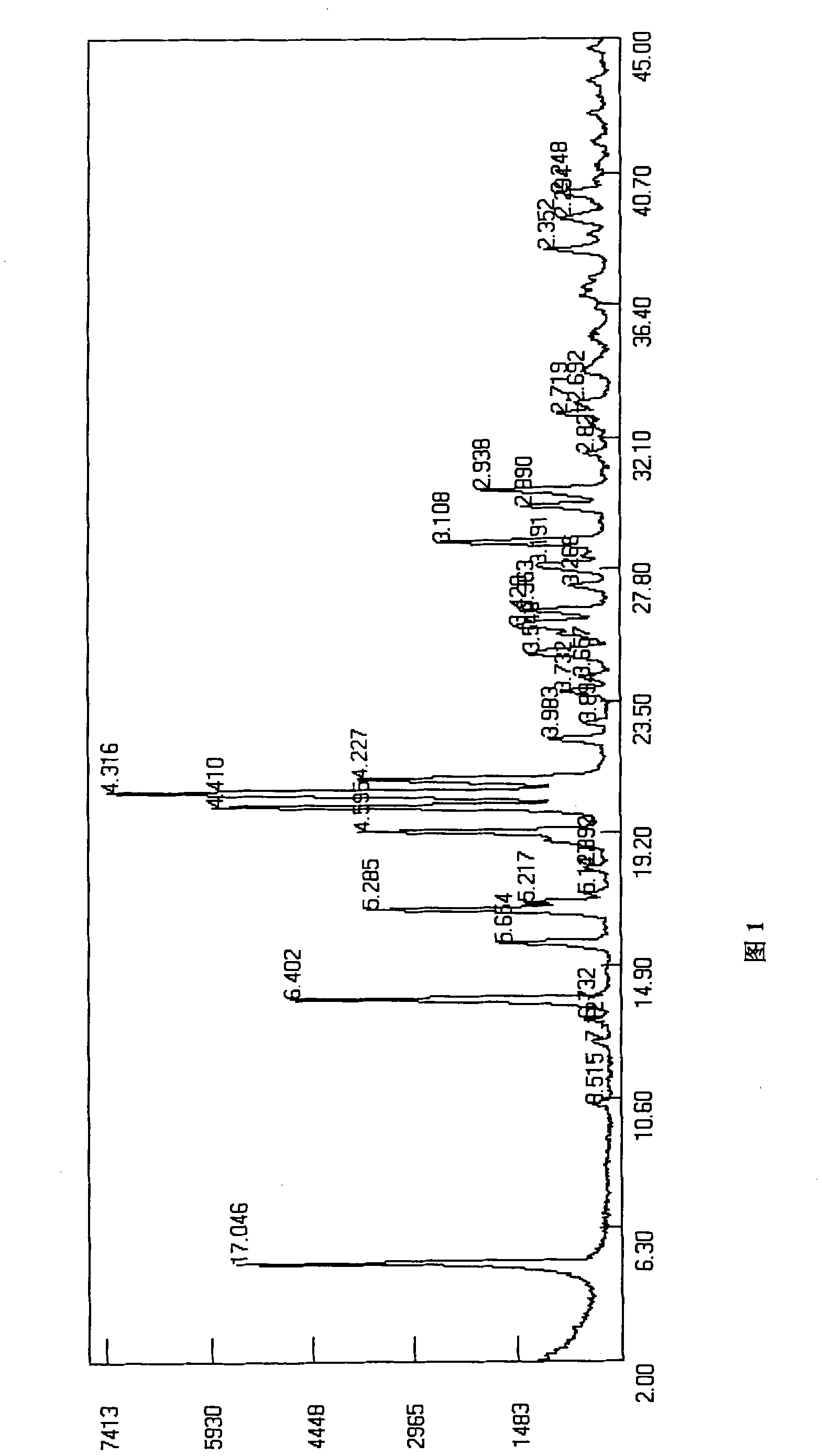
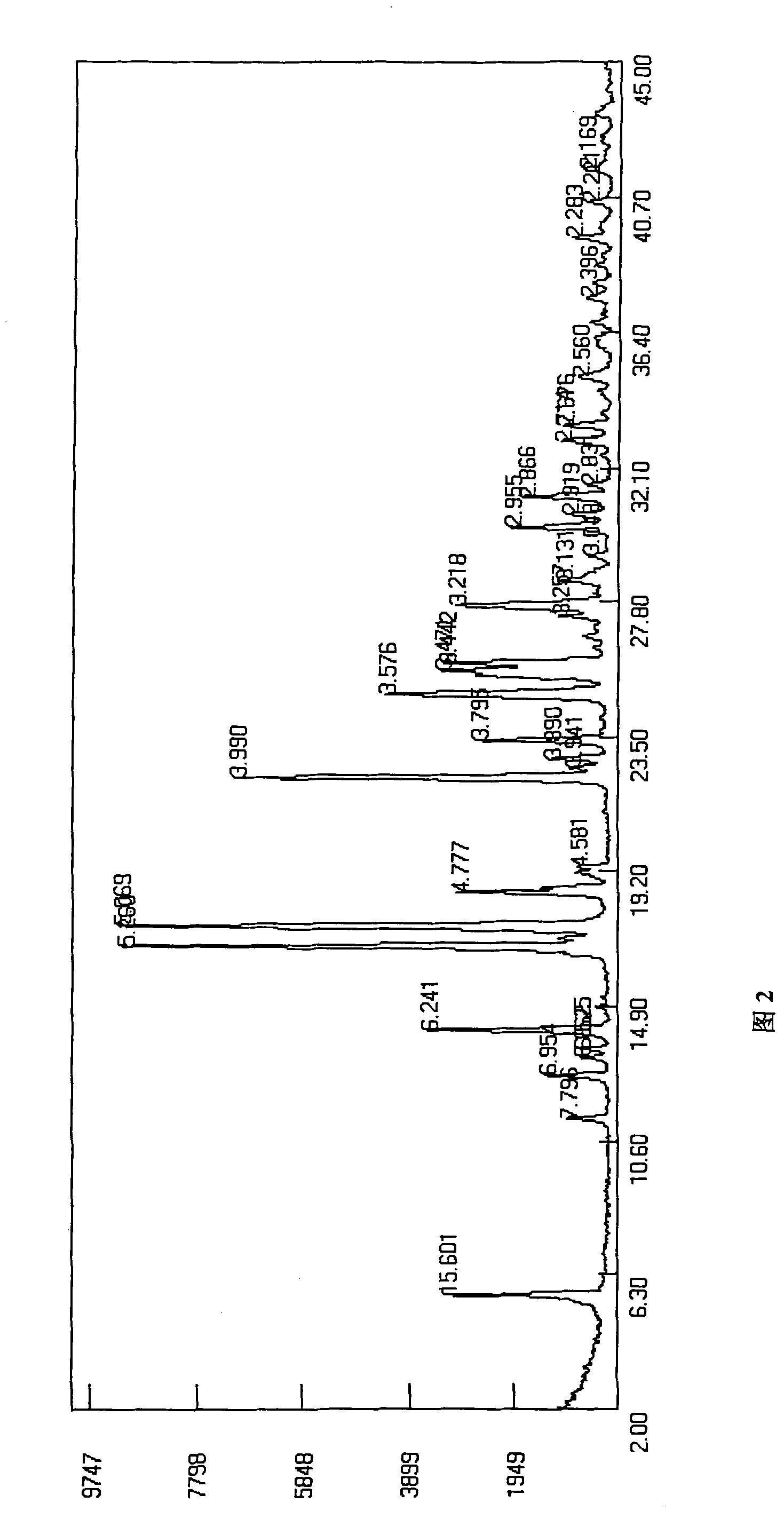
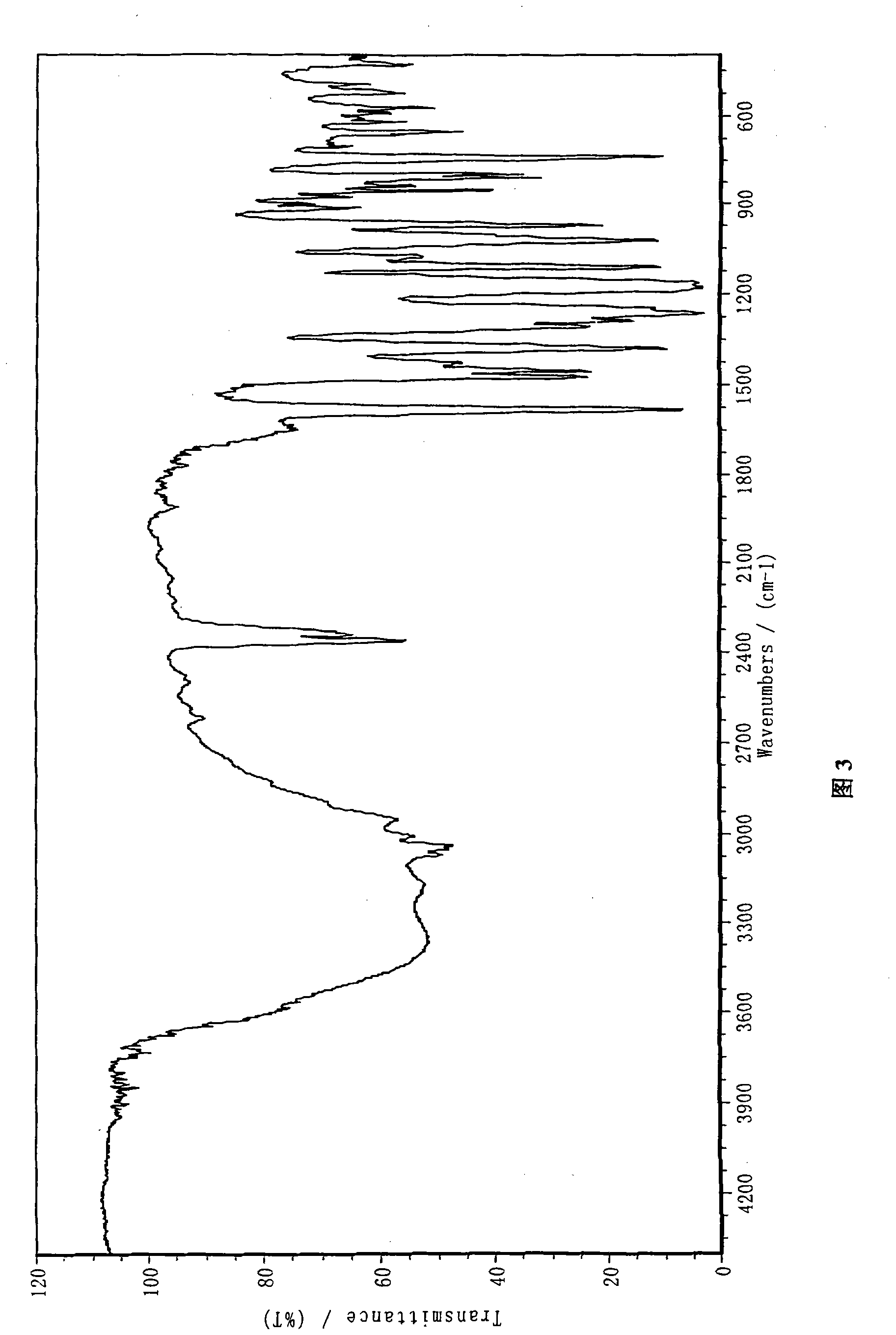
Lansoprazole sodium
A technology of lansoprazole sodium and acetone, applied in the B crystal form lansoprazole sodium and its preparation field, can solve the problems of lansoprazole sodium damage, product quality impact, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of B crystal form lansoprazole sodium:
[0033] Dissolve 5 g of crystal form A lansoprazole sodium in 15 ml of water, add 75 ml of acetone, stir evenly, then add 150 ml of diethyl ether dropwise to crystallize the solution, crystallize at about 10°C for 2 hours, filter, and dry under reduced pressure at 40°C to obtain crystal B Type lansoprazole sodium 4.5g, yield: 90%. HPLC detection content: 99.92%.
[0034] Attach the preparation method of A crystal form lansoprazole sodium:
[0035]A crystal form lansoprazole sodium can prepare lansoprazole sodium according to a general method such as being similar to the method (EP124495) for preparing omeprazole sodium from omeprazole or adopting the technology disclosed in patent CN1660092, CN1683367, CN1810245 .
[0036] Add 400ml of water and 21.6g of sodium hydroxide (0.54mol) to the reaction flask, stir to dissolve, cool to room temperature, add lansoprazole 200g (0.54mol), and stir until completely dissolv...
Embodiment 2
[0060] The preparation of B crystal form lansoprazole sodium:
[0061] Dissolve 5 g of crystal form A lansoprazole sodium in 10 ml of water, add 40 ml of acetone, stir evenly, then add 40 ml of diethyl ether dropwise to crystallize the solution, crystallize at about 10°C for 2 hours, filter, and dry under reduced pressure at 35°C for 4 hours to obtain B The crystalline form of lansoprazole sodium is 4.2 g, and the yield is 84%. HPLC detection content: 99.88%.
Embodiment 3
[0063] The preparation of B crystal form lansoprazole sodium:
[0064] Dissolve 5 g of crystal form A lansoprazole sodium in 5 ml of water, add 30 ml of acetone, stir evenly, then add dropwise 90 ml of diethyl ether to crystallize the solution, crystallize at about 0°C for 2 hours, filter, and dry under reduced pressure at 50°C for 4 hours to obtain B The crystalline form of lansoprazole sodium is 4.4g, and the yield is 88%. HPLC detection content: 99.86%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com