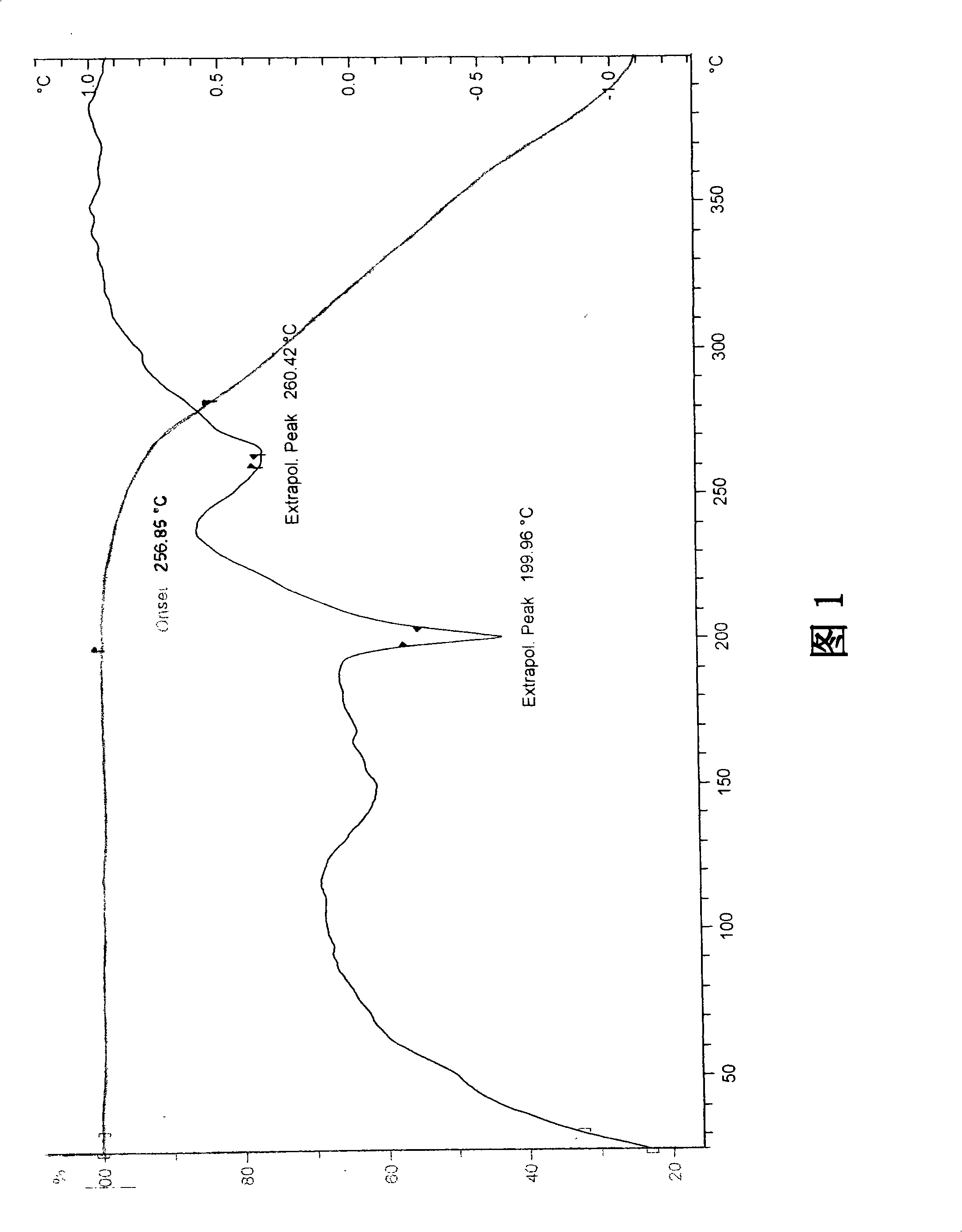
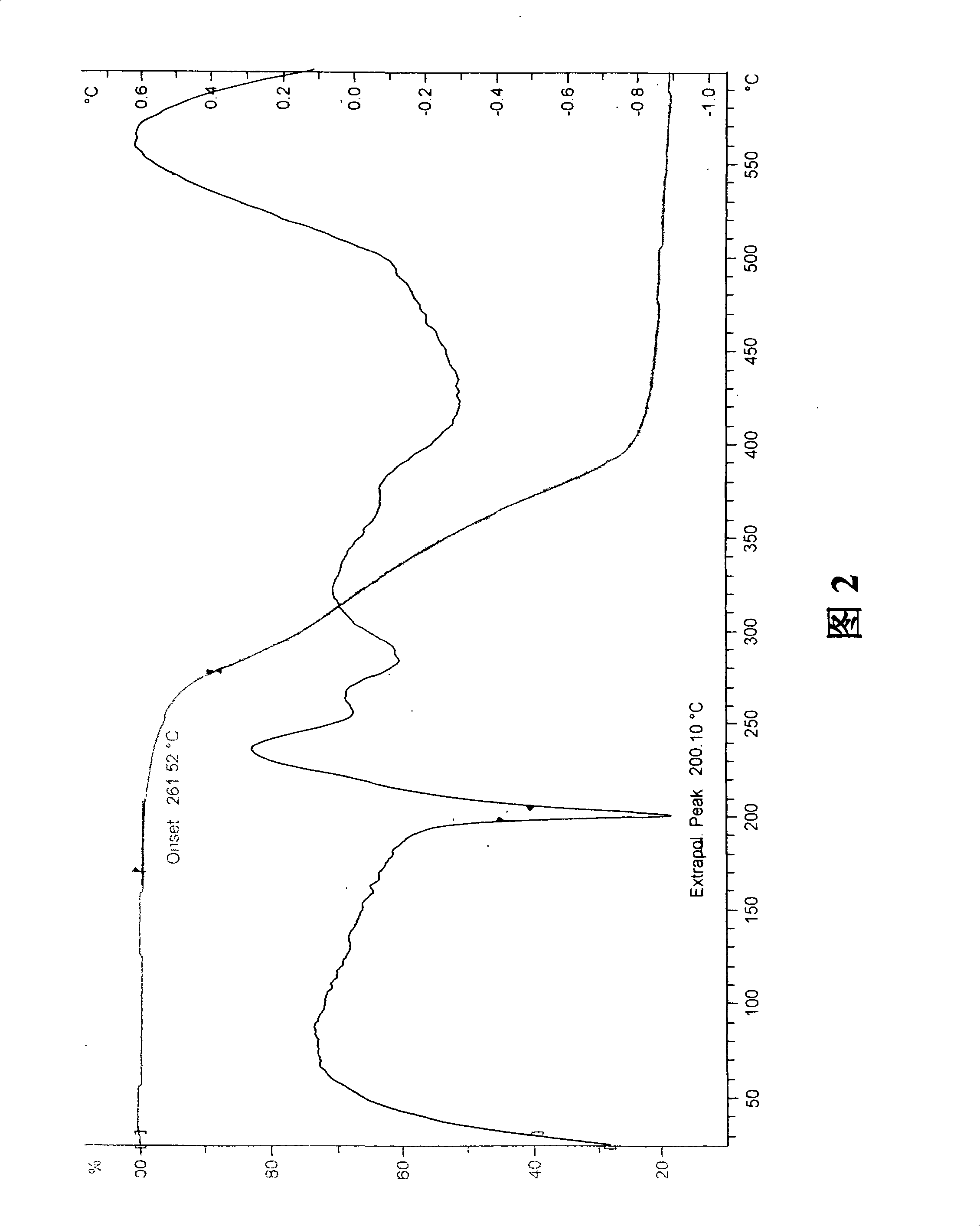
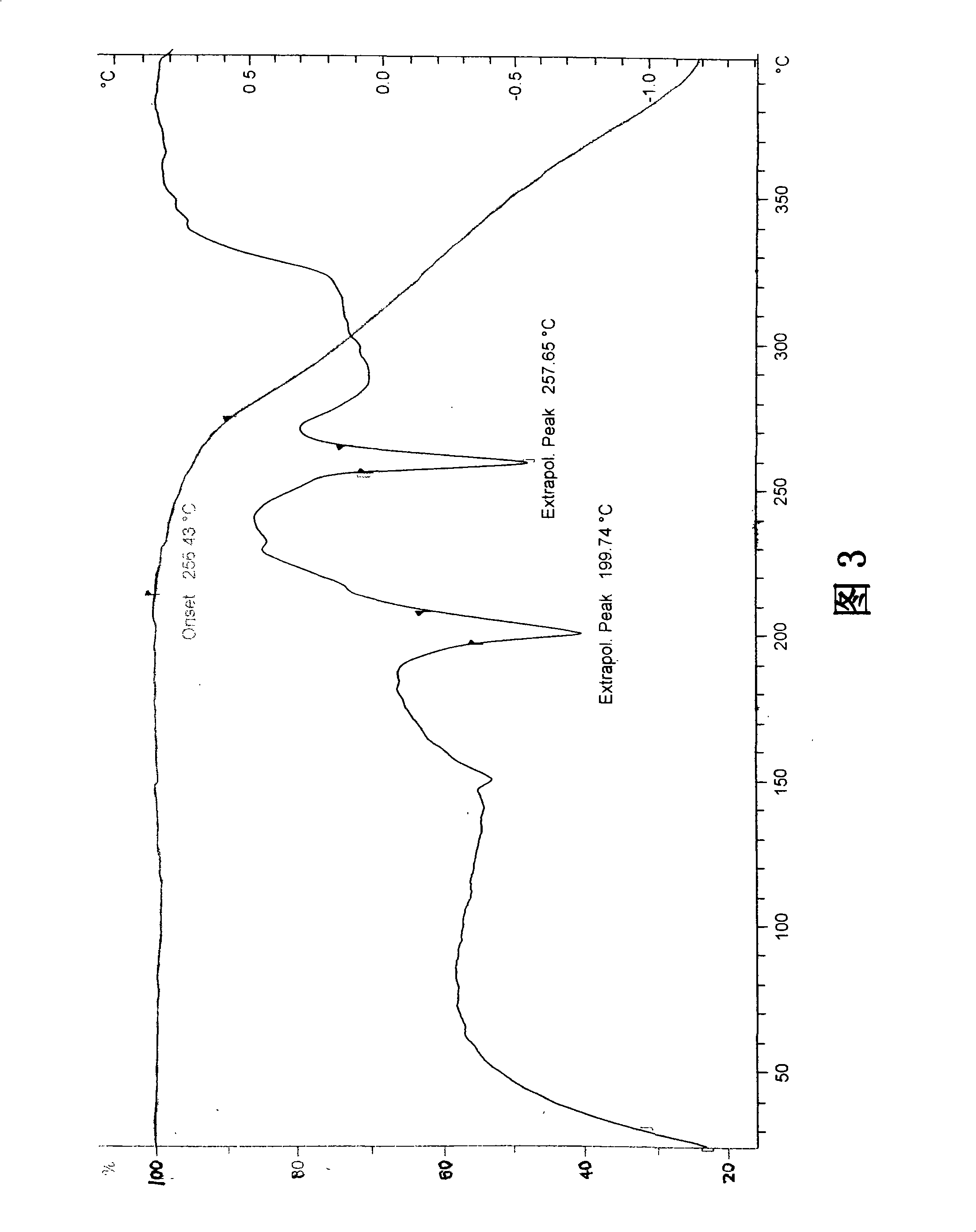
Stable Ivabradine crystal and preparation thereof
A technology of ivabradine hydrochloride and stable type, which is applied in the field of crystallization of stable ivabradine hydrochloride and its preparation, can solve the problems of unstable crystal form, unfavorable preparation processing, high boiling point of N-methylpyrrolidone, etc. and humidity stability, high superiority effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Heat and dissolve the mixture of ivabradine hydrochloride 5g and 400ml methyl ethyl ketone, then slowly cool down to the next day until the crystallization is complete, vacuum filter the crystals, and dry them in vacuum at 60°C to obtain off-white crystals, mp: 193-196 ℃, it begins to decompose above 210℃.
Embodiment 2
[0052] Heat 350ml of methyl ethyl ketone to 70°C, then add 5g of ivabradine hydrochloride in batches, reflux and stir until dissolved, then slowly cool to the next day until the crystallization is complete, vacuum filter out the crystals, and vacuum at 70°C Dry to obtain off-white crystals, mp: 192-195°C, and begin to decompose above 210°C.
[0053] X-ray Powder Diffraction Spectrum Data of Crystallized Product with Butanone (1)
[0054] 【F762.raw】YFBLD(DT)
[0055]
[0056]
Embodiment 3
[0058] Heat 6g of ivabradine hydrochloride and 15ml of N-methylpyrrolidone to dissolve, then slowly add 30ml of ethyl acetate dropwise under stirring, stir evenly, then slowly cool until the crystallization is complete, vacuum filter out the crystals, and use ethyl acetate Wash and dry under vacuum at 70°C to obtain colorless crystals, mp: 193-196°C. It begins to decompose above 210°C.
[0059] X-ray powder diffraction pattern data of crystallization product with N-methylpyrrolidone and ethyl acetate
[0060] 【D349.raw】YFBLD
[0061]
[0062]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com