Pharmaceutical composition for treating chronic stable angina pectoris with hyperlipemia
A technology for stable angina pectoris and hyperlipidemia, applied in the direction of drug combinations, non-active ingredients of polymer compounds, active ingredients of heterocyclic compounds, etc., can solve the problems of angina pectoris, the potential danger of ineffective suppression of angina pectoris, and the treatment of angina pectoris, etc. Achieve the effects of increasing intake and catabolism, good hemorheology effect, and good blood lipid-lowering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0034] One, embodiment 1~5: tablet composition
[0035] Component Example 1 2 3 4 5
[0036] Ivabradine hydrochloride 2.5 10 5 7.5 5
[0037] Atorvastatin Calcium 10 50 75 2.5 100
[0038]β-Cyclodextrin 10 30 40 20 50
[0039] Water 40 60 80 40 100
[0040] Microcrystalline cellulose 10 20 25 20 40
[0041] Compressible starch 20 40 20 30 40
[0042] Magnesium stearate 1 1 1 1 1
[0043] The above formula is the content of 1000 tablets, and the unit is gram.
[0044] Preparation method: Grind ivabradine hydrochloride, atorvastatin calcium, 3-cyclodextrin and water at high speed for 1 hour, then dry in vacuum at 40°C, pulverize, pass through a 100-mesh sieve, add the remaining materials, and mix in a mixer for 10 Minutes, tableting, packaging after passing the test.
Embodiment 6~10
[0045] Two, embodiment 6~10: capsule composition
[0046] Component Example 6 7 8 9 10
[0047] Ivabradine hydrochloride 2.5 10 5 7.5 5
[0048] Rosuvastatin Calcium 5 2.5 100 20 75
[0049] β-Cyclodextrin 20 30 50 40 35
[0050] water 40 10 30 20 40
[0051] Starch 240 200 250 220 230
[0052] Magnesium stearate 2 6 10 8 4
[0053] The above-mentioned formula is the content of 1000 grains, and the unit is gram.
[0054] Preparation method: Grind ivabradine hydrochloride, rosuvastatin calcium, β-cyclodextrin and water at high speed for 1 hour, then dry in vacuum at 40°C, pulverize, pass through a 100-mesh sieve, add the remaining raw materials, and mix in a mixer for 10 Minutes, filling capsules, packaging after passing the test.
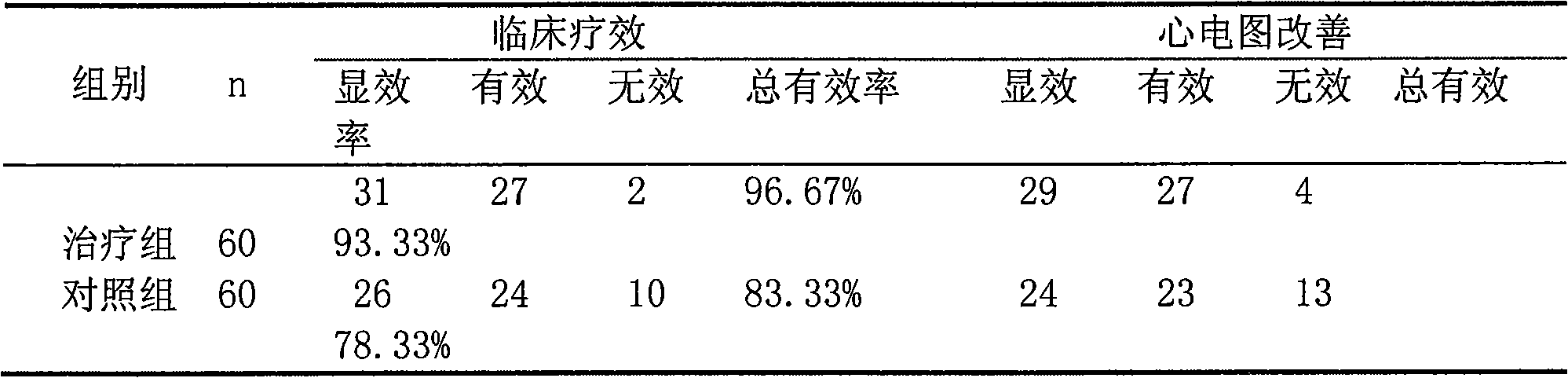
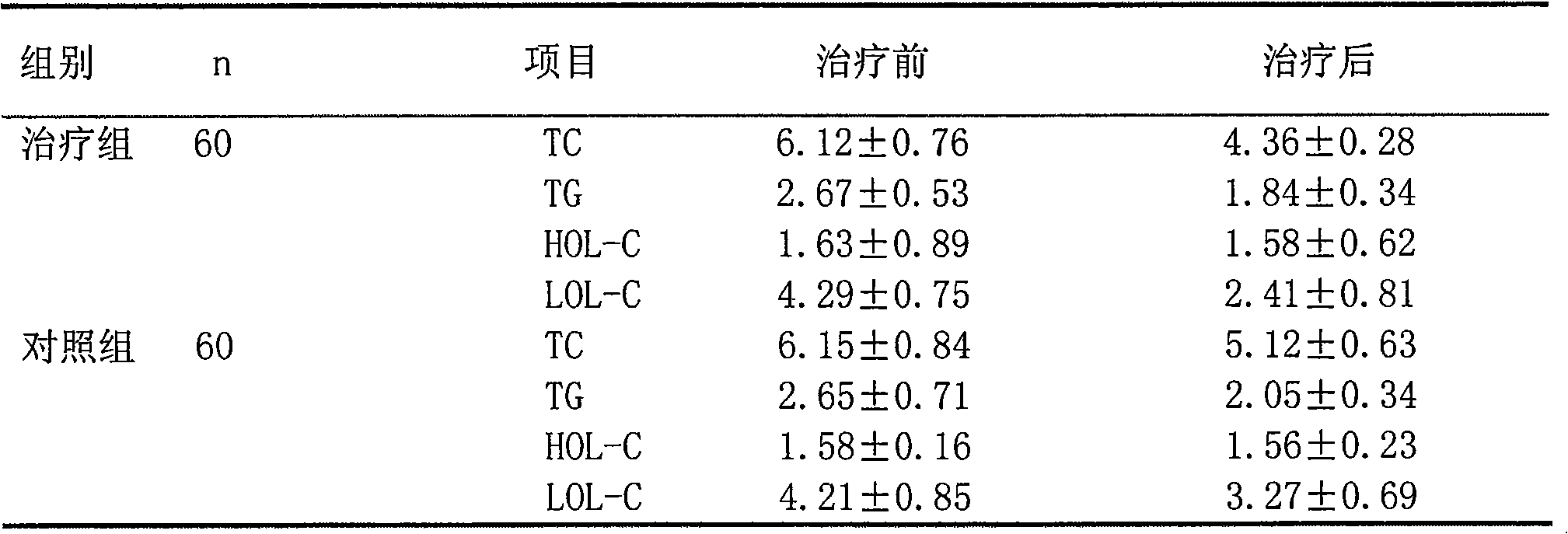
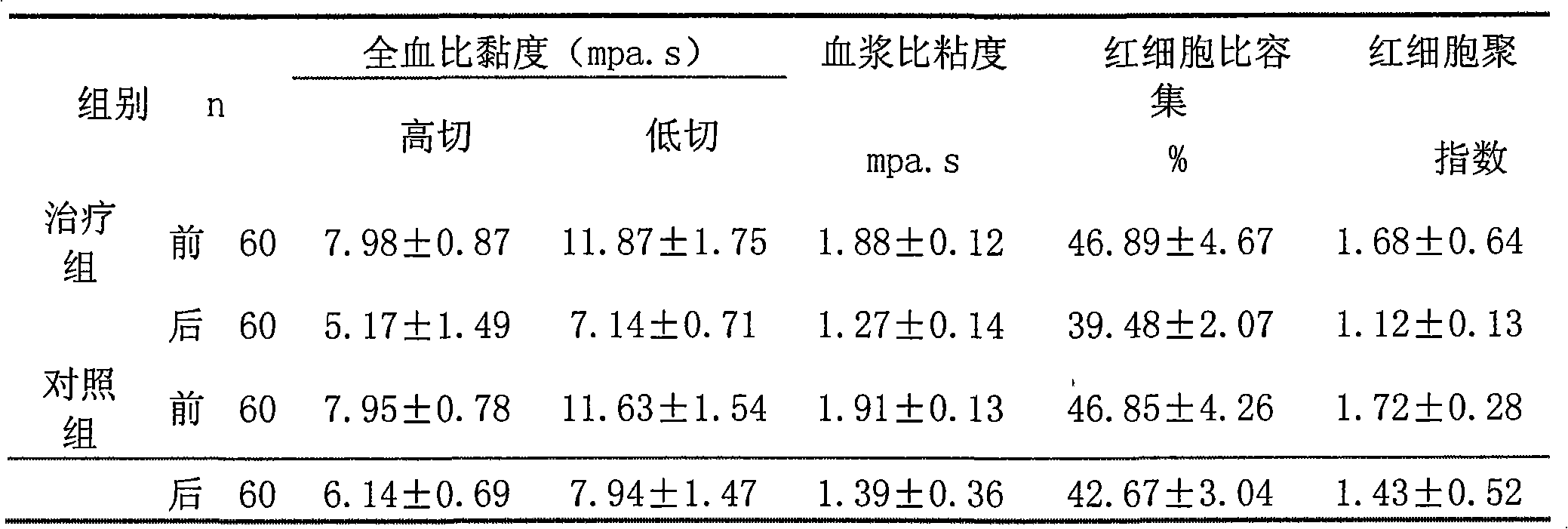
[0055] 3. Clinical trial of combination of atorvastatin calcium and ivabre hydrochloride in the treatment of stable angina pectoris complicated with hyperlipidemia
[0056] 1 Materials and methods
[0057] 1.1 The clinical data and diagnosi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com