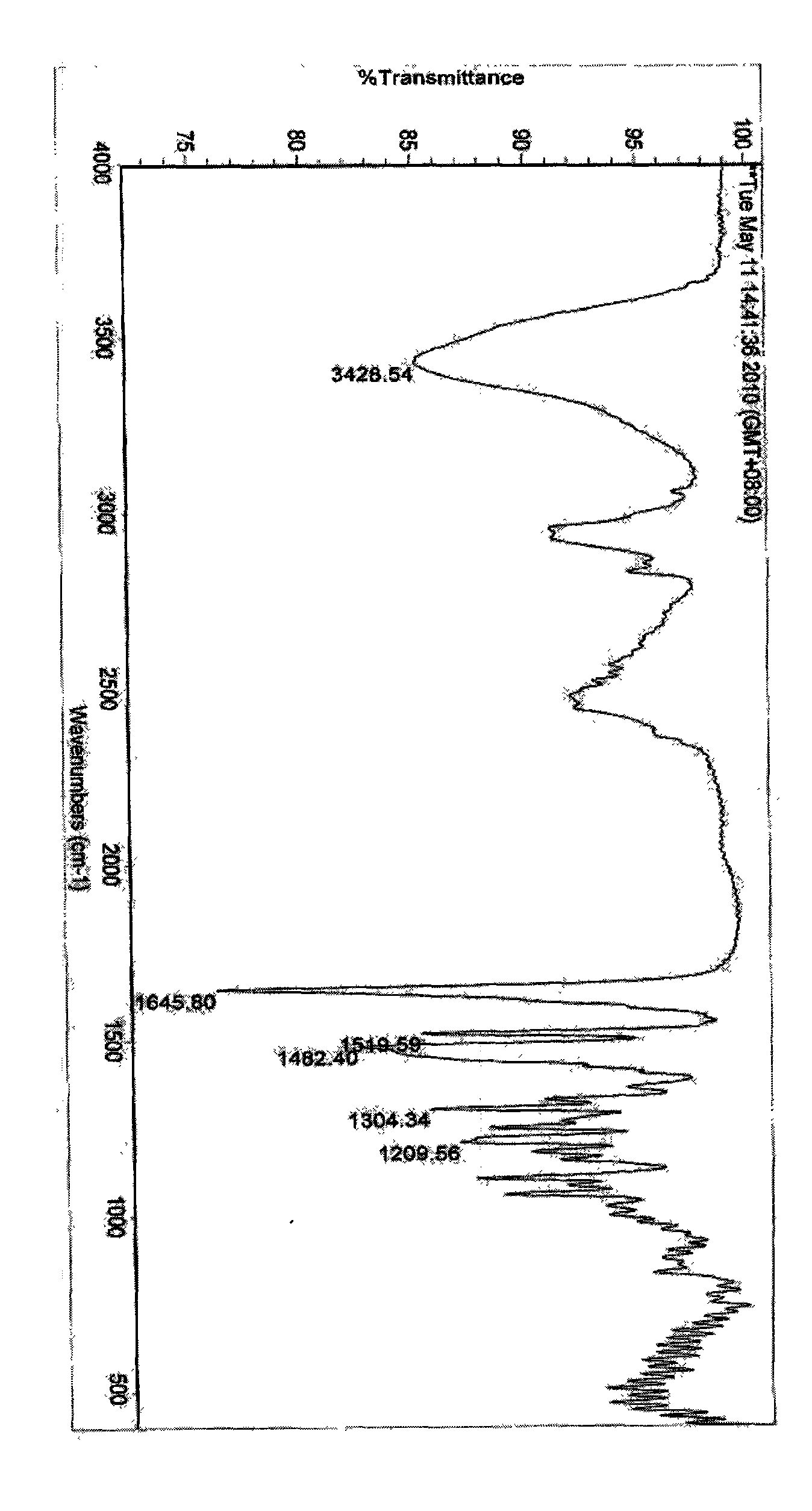
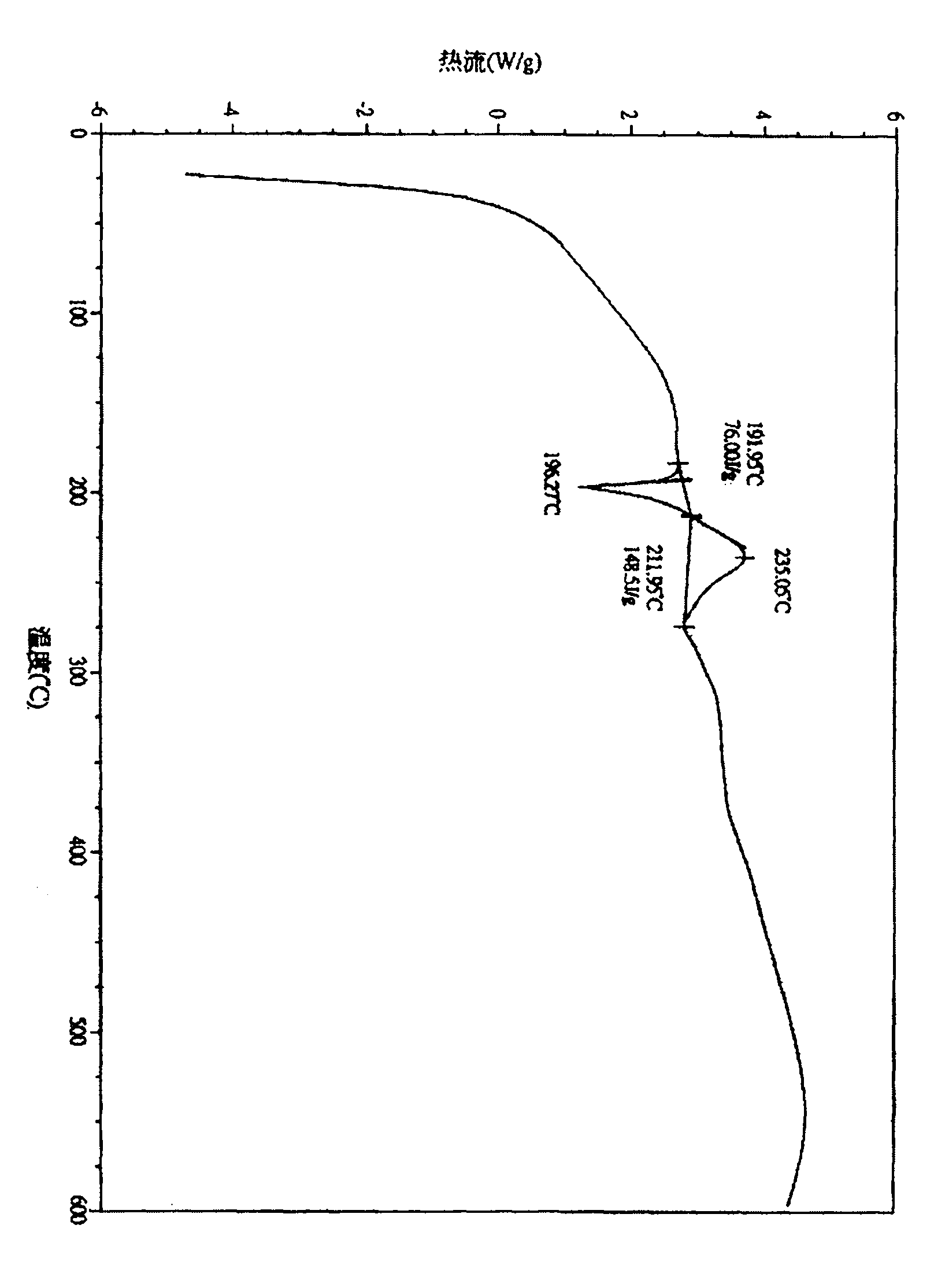
Novel ivabradine hydrochloride crystal form and its preparation method and use in preparation of pharmaceutical composition
A technology of ivabradine hydrochloride and crystal form, which is applied in the field of medicine to achieve the effect of being conducive to storage, high stability of temperature, humidity and light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Heat 100 g of ivabradine hydrochloride with 200 ml of anhydrous ethanol and stir until all is dissolved, stop heating, cool and stir to crystallize, after natural cooling to 21°C, stir and crystallize in an ice water bath at 1°C for 2 hours. After the crystallization is completed, the filter cake is washed with a small amount of absolute ethanol, and the filter cake is dried in a vacuum drying oven at 65°C to a constant weight to obtain a new crystal form of ivabradine hydrochloride with a purity of greater than 99.90%.
Embodiment 2
[0034] Heat 100g ivabradine hydrochloride with 400ml absolute ethanol under reflux and stir until all is dissolved, stop heating, cool and stir to crystallize, after natural cooling to 26°C, stir and crystallize in an ice water bath at 0°C for 3 hours. After the crystallization is completed, the filter cake is eluted with a small amount of absolute ethanol, and the filter cake is dried to a constant weight in a vacuum drying oven at 70°C to obtain a new crystal form of ivabradine hydrochloride with a purity of greater than 99.89%.
Embodiment 3
[0036] Heat 100 g of Ivabradine hydrochloride with 800 ml of ethyl acetate and absolute ethanol (3:1) and stir under reflux until all is dissolved. Stop heating, cool and stir to crystallize. After natural cooling to 25°C, stir under 1°C ice water bath Crystallize for 2h. After the crystallization is completed, the filter cake is washed with a small amount of ethyl acetate, and the filter cake is dried to constant weight in a vacuum drying oven at 71° C. to obtain a new crystal form of ivabradine hydrochloride with a purity of greater than 99.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com