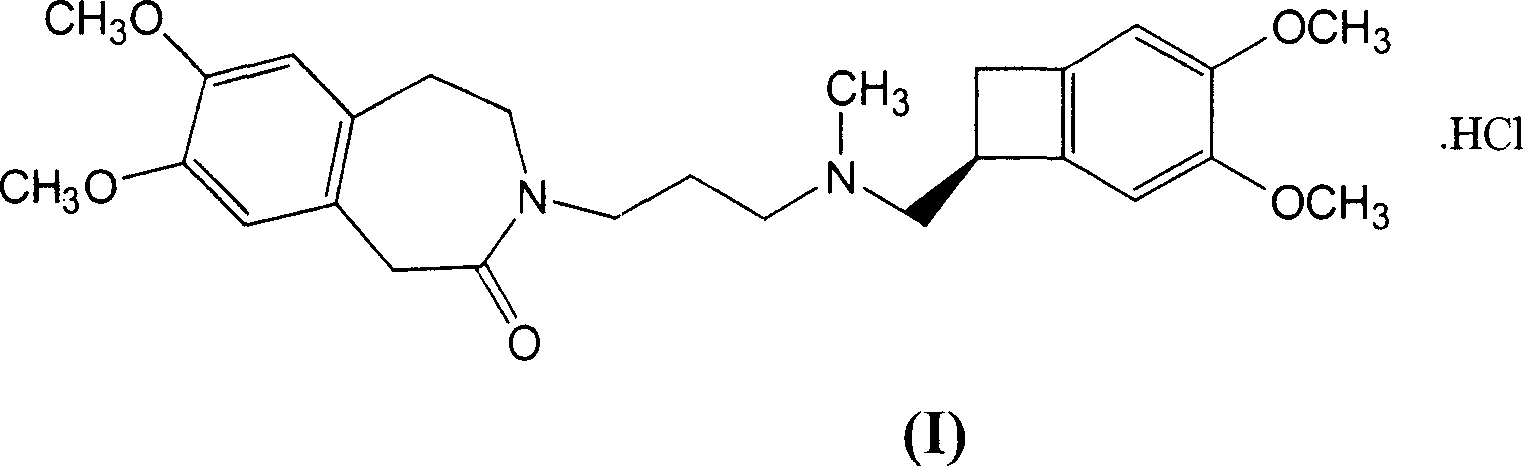
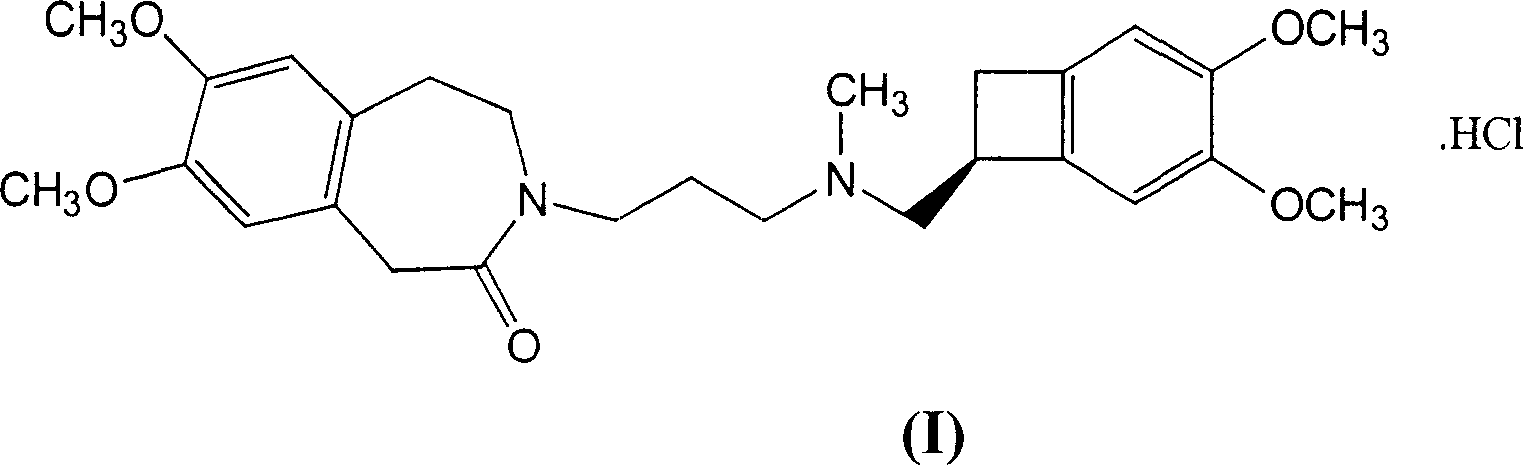
Delta,-crystalline form of ivabradine hydrochloride, a process for its preparation and pharmaceutical compositions containing it
A technology of ivabradine hydrochloride and crystallization, which is applied in the directions of drug combination, organic chemistry method, active ingredient of heterocyclic compounds, etc., can solve the problems of unspecified acquisition and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Ivabradine hydrochloride in delta-crystalline form
[0034] 160ml of acetonitrile was preheated to 70°C, then 2g of ivabradine hydrochloride obtained by the method described in the patent specification EP0534859 was added part by part, while stirring until completely dissolved. The solution was then kept at ambient temperature for 2 days. Crystals were vacuum filtered and spread on crystallization trays.
[0035] The water content of the obtained product was determined by coulometric method to be 2.8%.
[0036] Powder X-ray Diffraction Pattern:
[0037] The powder X-ray diffraction pattern (diffraction angles) of the delta-form of ivabradine hydrochloride is given in the table below with highlighted lines:
[0038]
[0039] 4
Embodiment 2
[0040] Embodiment 2: pharmaceutical composition
[0041] Formulation for the preparation of 1000 tablets each containing 5 mg ivabradine base:
[0042] Compound of Example 1 ................................. 5.39g
[0043] corn starch................................................ .20g
[0044] Anhydrous colloidal silica...................................0.2g
[0045] Mannitol................................................ 63.91g
[0046] PvP................................................................ .....10g
[0047] Magnesium stearate.............................................. ..0.5g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com