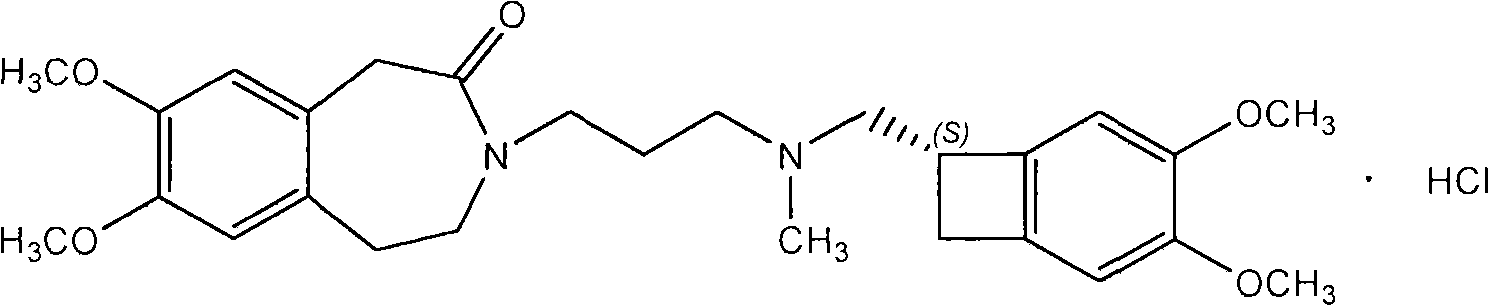
Novel method for preparing amorphous Ivabradine hydrochloride
An ivabradine hydrochloride and amorphous technology, which is applied in the field of preparation of amorphous ivabradine hydrochloride, can solve the problems of low production efficiency and achieve the effects of improving production efficiency, high yield and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 100g of ivabradine hydrochloride is added in 1000ml of ethanol, stirred to dissolve. The resulting solution was added into 4000ml of ether to obtain a mixed solution, which was stirred at a high speed at 15°C-30°C. The resulting crystalline slurry was stirred for 1 hour. The crystalline slurry was cooled to about 0° C., stirred for 2 hours, filtered, and dried to collect 81 g of amorphous ivabradine hydrochloride with a yield of 81.0%.
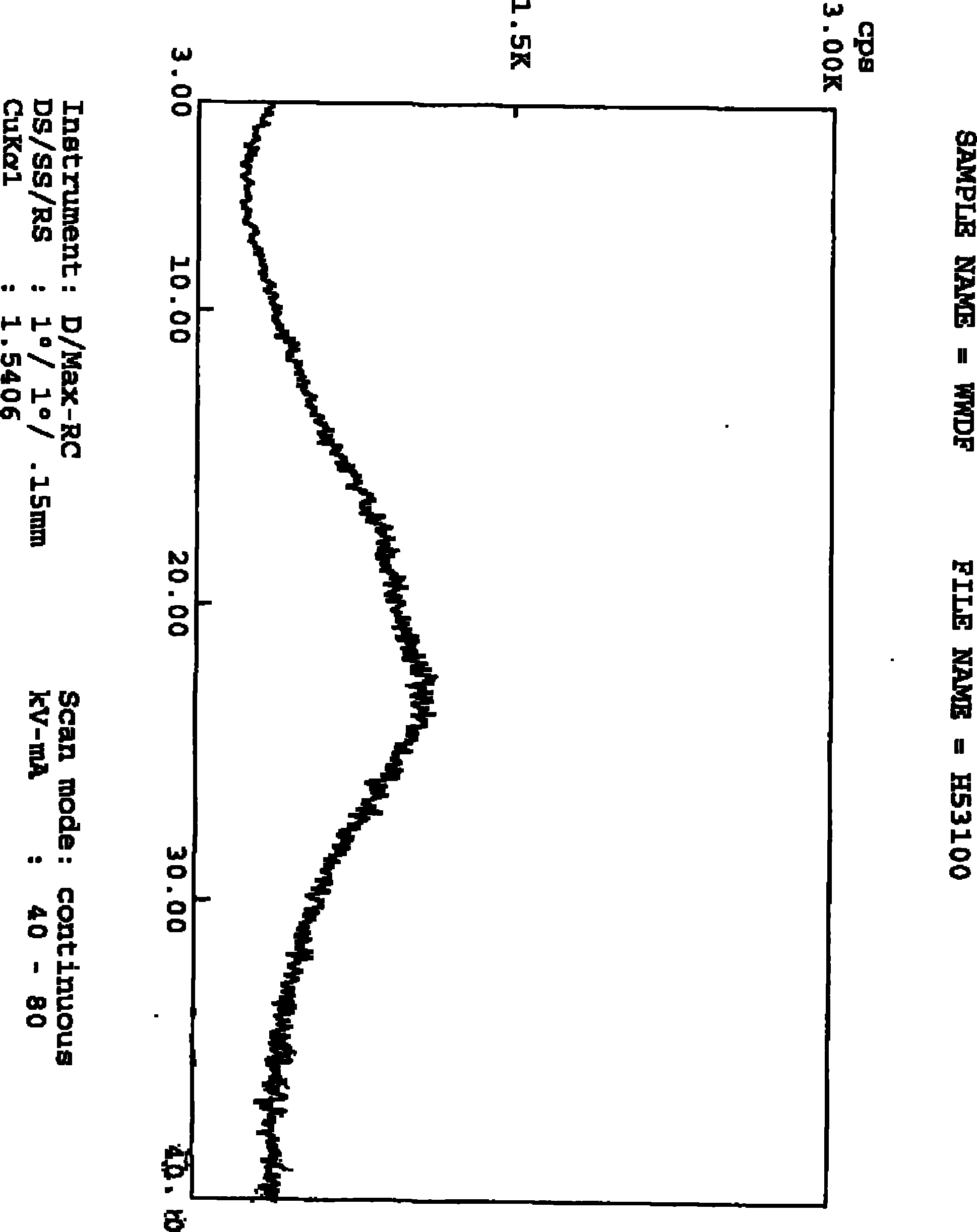
[0023] The powder X-ray diffraction pattern of amorphous ivabradine hydrochloride is shown in the attached Figure 1 .
Embodiment 2
[0025] 100g of ivabradine hydrochloride is added in 1000ml of acetone, stirred to dissolve. The resulting solution was added into 3000ml of cyclohexane to obtain a mixed solution, which was stirred at a high speed at 15°C-30°C. The resulting crystalline slurry was stirred for 1 hour. The crystalline slurry was cooled to about 0° C., stirred for 2 hours, filtered, and dried to collect 83 g of amorphous ivabradine hydrochloride with a yield of 83.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com