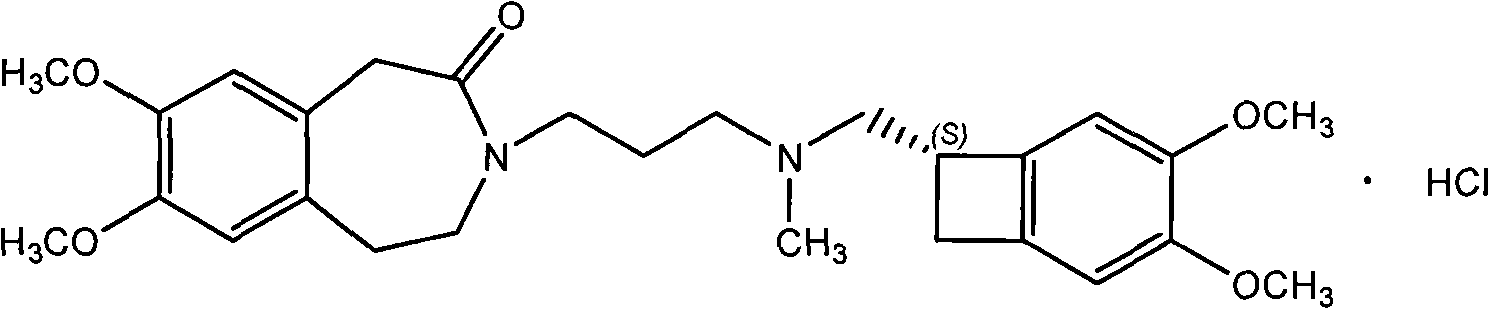
Amorphous ivabradine hydrochloride
An amorphous technology of ivabradine hydrochloride, applied in the fields of active ingredients of heterocyclic compounds, cardiovascular system diseases, organic chemistry, etc., can solve problems such as high boiling point, non-specific crystal form, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 amorphous ivabradine hydrochloride
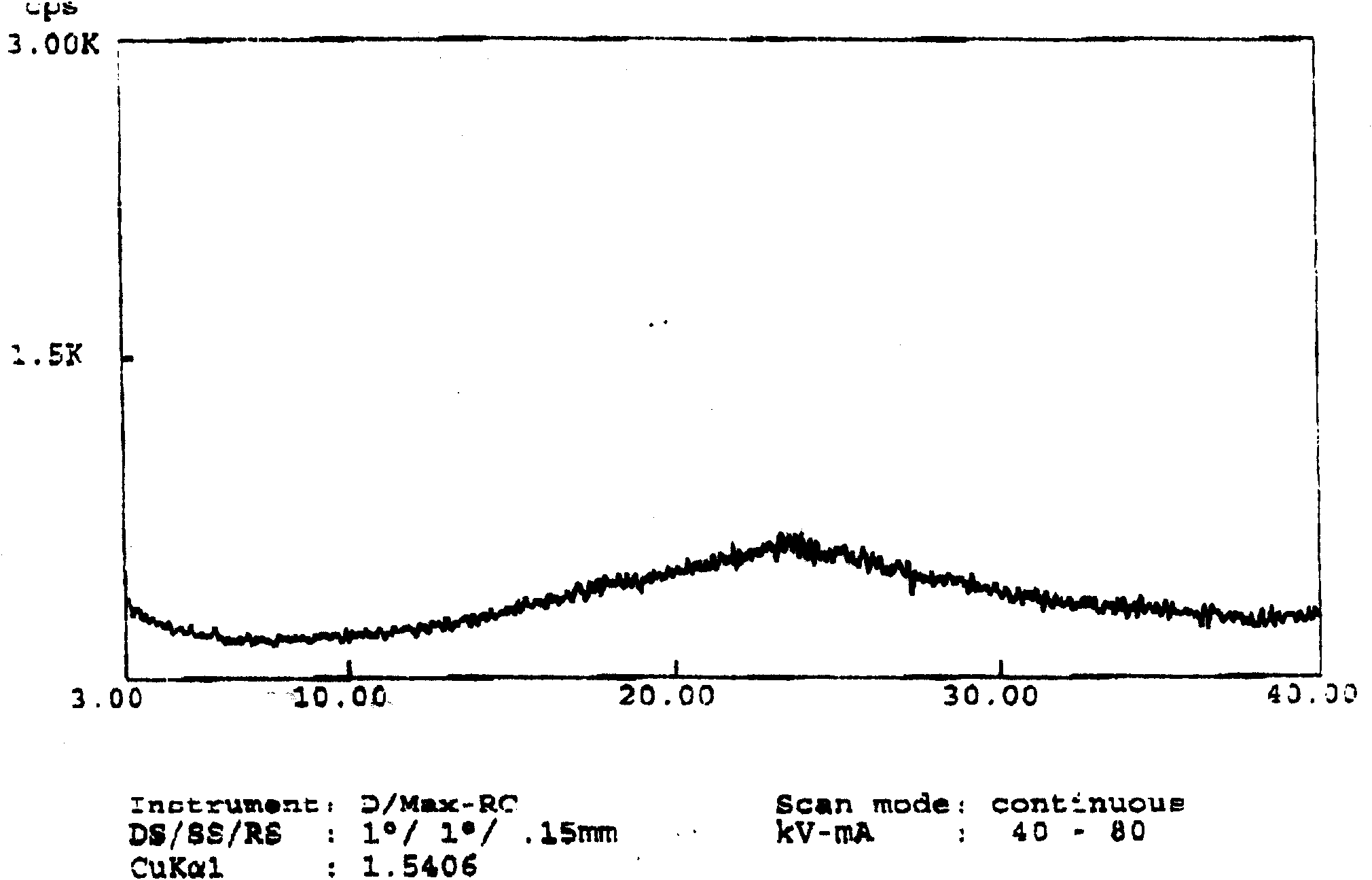
[0021] 30 grams of ivabradine hydrochloride was dissolved in 500 milliliters of water to prepare ivabradine hydrochloride aqueous solution. Control the external temperature in a water bath to be less than 40°C, and distill to dryness under reduced pressure to obtain a white solid, which is detected by X-ray powder diffraction spectrum and is amorphous ivabradine hydrochloride.
Embodiment 2
[0022] The preparation of embodiment 2 amorphous ivabradine hydrochloride
[0023] 30 grams of ivabradine hydrochloride was dissolved in 300 milliliters of anhydrous methanol to prepare ivabradine hydrochloride methanol solution. Control the external temperature in a water bath to be less than 40°C, and distill to dryness under reduced pressure to obtain a white solid, which is detected by X-ray powder diffraction spectrum and is amorphous ivabradine hydrochloride.
Embodiment 3
[0024] The preparation of embodiment 3 amorphous ivabradine hydrochloride
[0025] Dissolve 10 grams of ivabradine hydrochloride in 100 milliliters of ethanol (95) to prepare ivabradine hydrochloride ethanol solution. Control the external temperature in a water bath to be less than 40°C, and distill to dryness under reduced pressure to obtain a white solid, which is detected by X-ray powder diffraction spectrum and is amorphous ivabradine hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com