Preparation method for ivabradine and intermediate thereof
An ivabradine and dehydrogenation technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, catalytic reactions, etc., can solve the problem of large amount of organic solvent, long reaction time and high process cost The problem is to improve the yield and purity, avoid the column chromatography process, and have a good industrialization prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
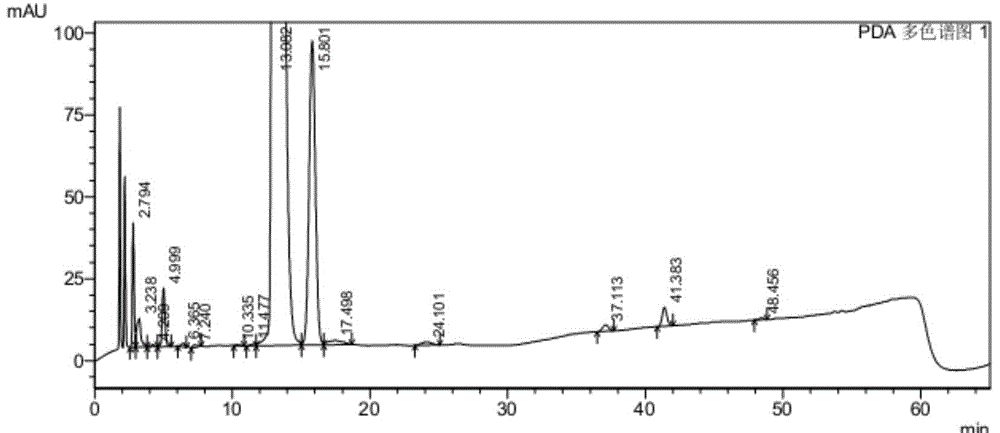
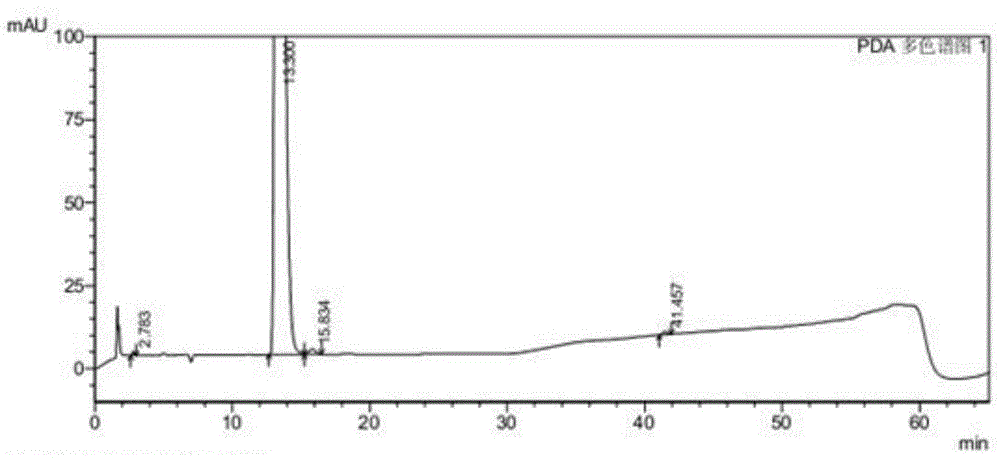
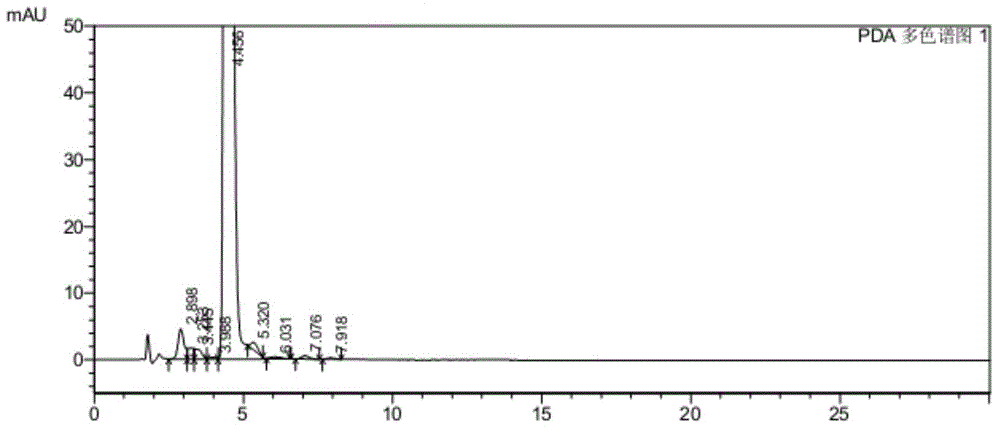
Image
Examples
Embodiment 1
[0055] Example 1 Research on Phase Transfer Catalysts
[0056] The inventors conducted research on commonly used phase transfer catalysts (as shown in Table 1). The end point of the nucleophilic substitution reaction is monitored by HPLC (high performance liquid chromatography), and the reaction is considered complete when the remaining amount of the compound shown in formula II (wherein the substituent X is Br) is less than 0.5%. The experimental results are shown in Table 1. It can be seen from Table 1 that when no phase transfer catalyst is used, the nucleophilic substitution reaction takes up to 15.5 hours; When the quaternary ammonium salt phase transfer catalyst is used as a single phase transfer catalyst, it has played a certain catalytic effect, and the reaction time is shortened to 9.5~13.0h, but the catalytic effect is still not ideal; use catalytic amount (2% of the compound quality shown in formula III %~10%) polyether phase transfer catalyst as a single phase t...
Embodiment 2
[0068] Example 2 Preparation of dehydroivabradine
[0069] In a 500mL three-neck reaction flask, dissolve 20.0g of the compound of formula III in 200mL of DMF, add 30.0g of anhydrous K 2 CO 3 , stirred for 30 minutes, then added 39.1g of formula II compound (wherein substituent X is Br), 0.8g composite phase transfer catalyst (mixed by benzalkonium bromide and polyethylene glycol-800 in a mass ratio of 5:1 ), the temperature of the reaction system was raised to 85° C., and the end point of the reaction was monitored by HPLC, and the reaction was complete after 3.0 h. After the reaction is completed, cool, collect the filtrate by filtration, add the filtrate to 500mL saturated sodium chloride solution, extract twice with 250mL and 200ml ethyl acetate respectively, combine the organic phases, add 20g of anhydrous NaCl 2 SO 4 After drying and filtering, the filtrate was concentrated under reduced pressure at 50° C. to obtain 37.9 g of dehydroivabradine oily substance with a ...
Embodiment 3
[0073] Example 3 Preparation of dehydroivabradine
[0074] In a 500mL three-necked reaction flask, 20.0g of the compound of formula III was dissolved in 200mL of DMF, and 23.0g of anhydrous Na 2 CO 3, stirred for 30 minutes, then added 27g of formula II compound (wherein substituent X is C1), 0.6g composite phase transfer catalyst (mixed by benzalkonium bromide and polyethylene glycol-200 at a mass ratio of 7: 1) , the temperature of the reaction system was raised to 80° C., and the end point of the reaction was monitored by HPLC, and the reaction was complete after 4.5 hours of reaction. After the reaction is complete, cool, collect the filtrate by filtration, add the filtrate to 500mL saturated sodium chloride solution, extract twice with 250mL and 200ml ethyl acetate respectively, combine the organic phases, add 20g of anhydrous NaCl 2 SO 4 After drying and filtering, the filtrate was concentrated under reduced pressure at 50° C. to obtain 37.7 g of dehydroivabradine o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com