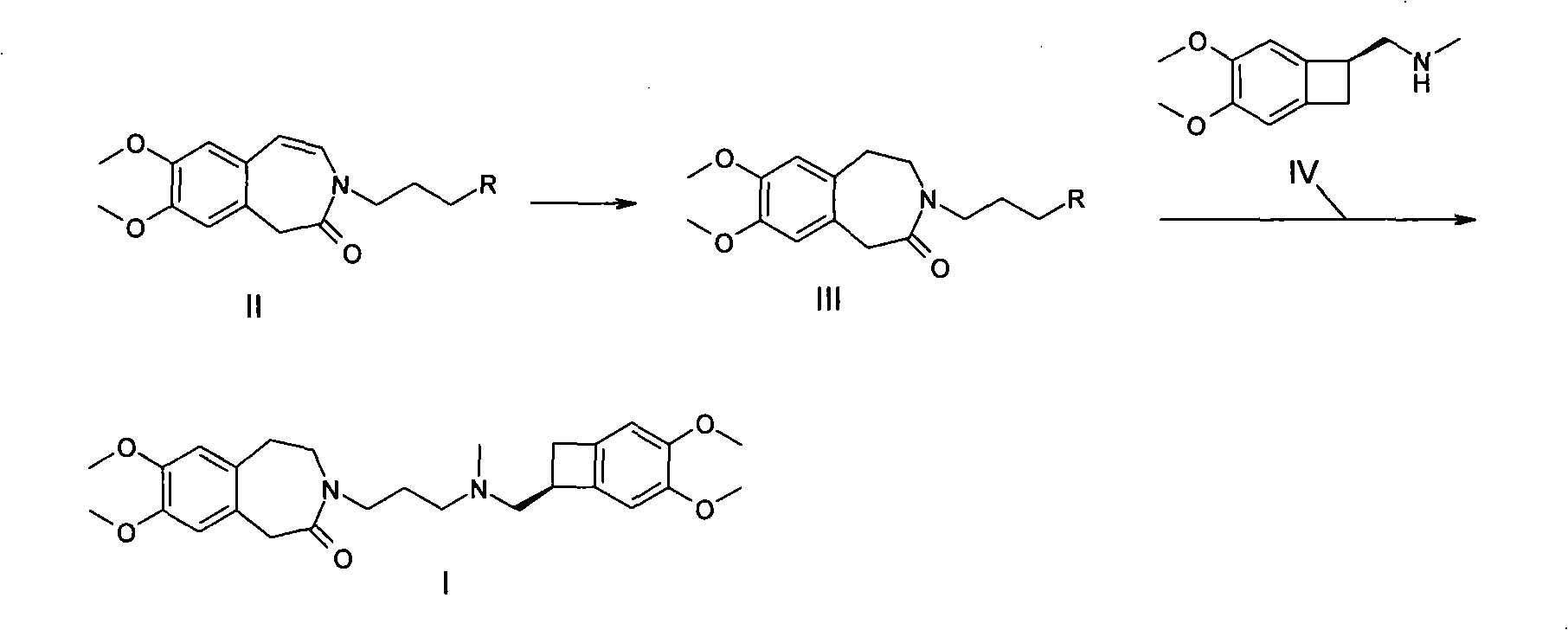
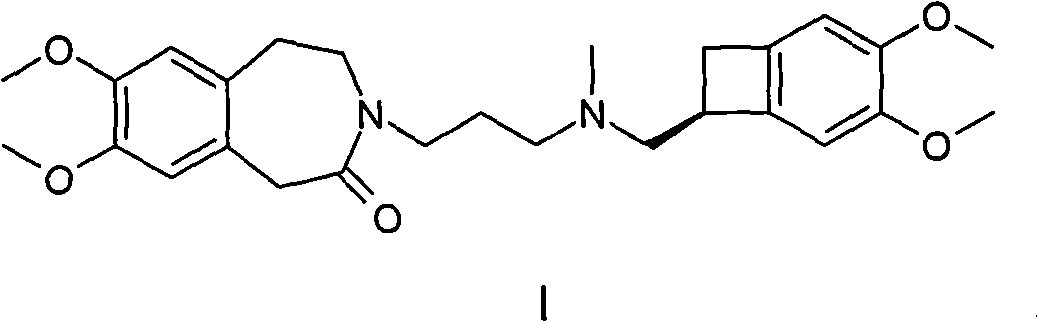
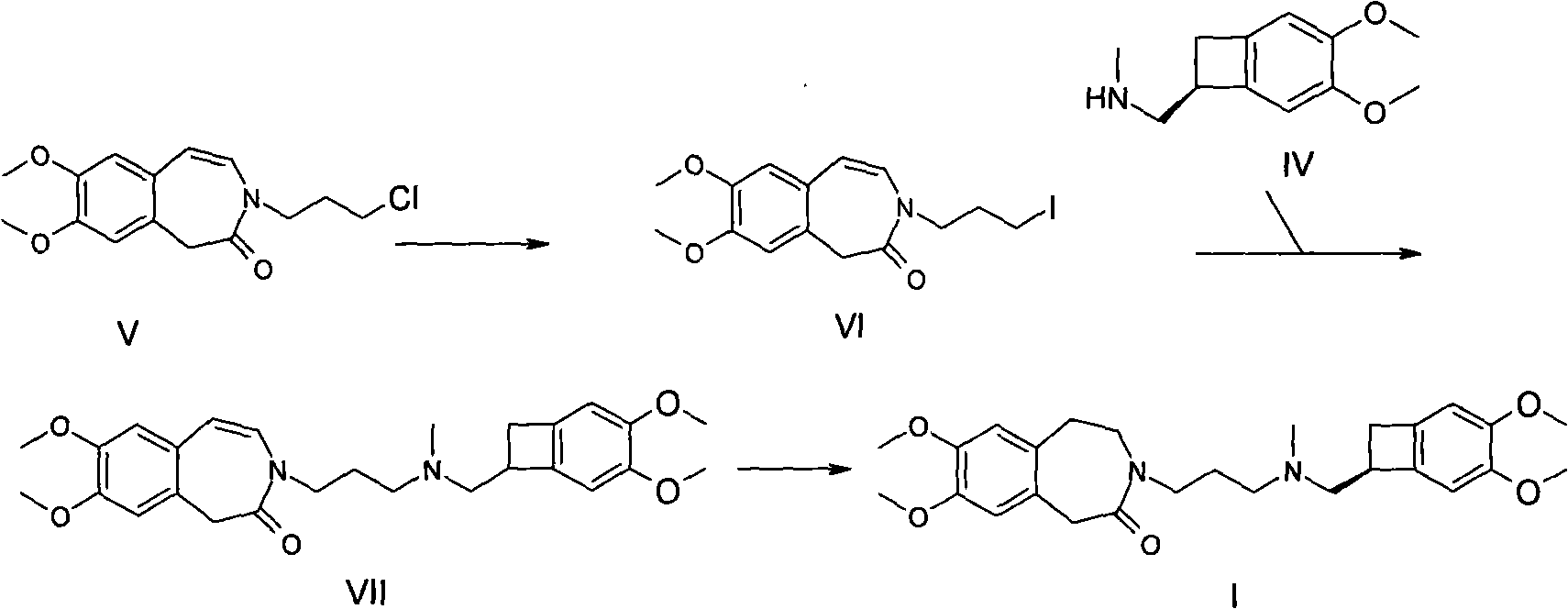
Preparation method of ivabradine
A technology of ivabradine and its compound, which is applied in the field of preparation of angina drug ivabradine and its salt, can solve the problems of high cost, complicated preparation process and high cost, and achieve simple method, easy-to-obtain raw materials and large application value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 7
[0030] Example 1, Synthesis of 7,8-dimethoxy-3-(3-chloropropyl)-1,3,4,5-tetrahydro-2H-3-benzazepin-2-one:
[0031] 7,8-dimethoxy-3-(3-chloropropyl)-1,3-dihydro-2H-3-benzazepine - 2-ketone (15.0g, 50.8mmol), ethanol (80ml) and 5% Pd / C (0.8g) were added to the autoclave, and reacted at 5-10 bar hydrogen pressure at 60°C until no hydrogen was absorbed, and the reaction was terminated , cooled, filtered, concentrated under reduced pressure to about 30ml, cooled and filtered to obtain 7,8-dimethoxy-3-(3-chloropropyl)-1,3,4,5-tetrahydro-2H-3-benzene And aza -2-Kone, white solid 13.9g, mp: 93-95°C, yield 92.1%
[0032] MS: 297(M+), 299(M+2), 262(M-Cl)
[0033] 1HNMR (CDCl3): δ2.06 (2H, m), 3.07 (2H, t), 3.56 (2H, M), 3.76 (2H, t), 3.81 (2H, t), 3.84 (3H, s), 3.85 (3H, s), 6.58-6.60 (2H, aromatic hydrogen)
Embodiment 2
[0034] Example two, 3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]oct-1,3,5-trien-7-yl]-methyl}( Methyl)amino]propyl-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine Synthesis of -2-ketone (compound 1, ivabradine):
[0035] 7,8-dimethoxy-3-(3-chloropropyl)-1,3,4,5-tetrahydro-2H-3-benzazepine -2-Kone (10.0g.33.7mmol), (1S)-4,5-dimethoxy-1-(methylaminomethyl)-benzocyclobutane (compound IV, 7.0g, 33.8mmol) , triethylamine (10ml) and acetonitrile (50ml) were added into a three-necked flask, then stirred at room temperature for 20 hours, then concentrated under reduced pressure to 200ml of water, extracted with ethyl acetate (150ml*3), dried over anhydrous magnesium sulfate , filtered, and concentrated under reduced pressure to obtain a yellow oil, namely 3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]oct-1,3,5-triene-7 -yl]-methyl}(methyl)amino]propyl-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine -2-one, about 13.5 g.
[0036] The sample for analysis was obtained by silica gel col...
Embodiment 3
[0039] Example three, 3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]oct-1,3,5-trien-7-yl]-methyl}( Methyl)amino]propyl-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine Preparation of -2-one hydrochloride:
[0040] The compound 3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]oct-1,3,5-trien-7-yl]-methyl}(methyl )amino]propyl-7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepine -2-Kone (ivabradine) (13.5g,) was dissolved in acetonitrile (40ml), then heated to 70°C, and under stirring, dry HCl gas was introduced until the pH of the reaction solution was about 2-3, and 1 g of activated carbon was added, Reflux for 10 minutes, filter while hot, cool, filter, and vacuum dry at 60°C to obtain 12.4g of a near-white powder, mp:°C, yield 73.1% (calculated as compound III), and recrystallize the above crude product with acetonitrile to obtain 10.6g of a white powder , yield 85.5%, HPLC > 99.0%, [α] 7.2 (DMSO, 1%)
[0041] MS: 468(M+), 469(M+1), 453(M-CH3), 305(M-163), 262(M-206).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com