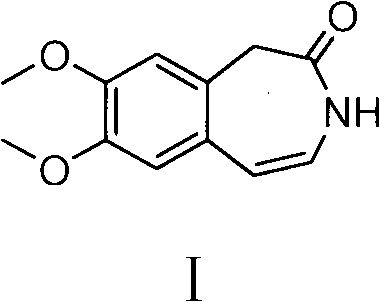
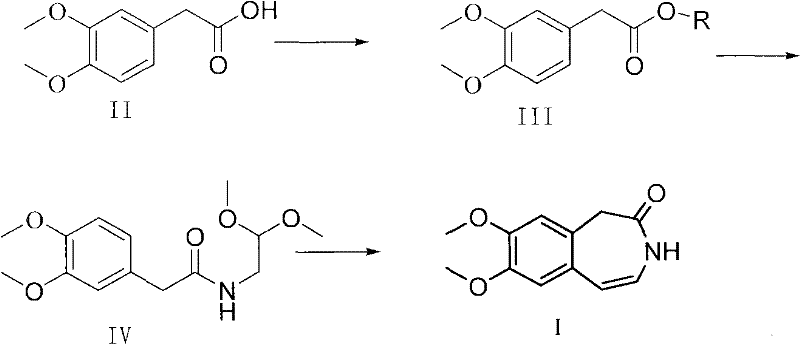
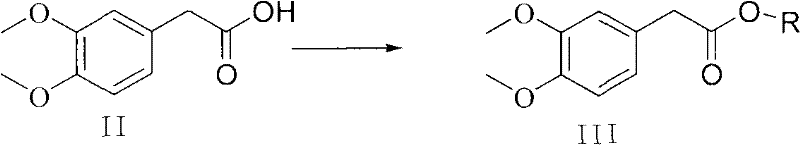
Preparation method of 7,8-dimethoxy-1,3-dihydro-2h-3-benzazepin-2-one
A technology of -2H-3- and dimethoxy, which is applied in the direction of organic chemistry, can solve the problems of equipment corrosion, environmental pollution, unfavorable industrial production, and reduced production efficiency, so as to avoid high-temperature vacuum distillation and shorten the reaction The effect of simple time and production conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 2,4-dimethoxy-6-chloro-1,3,5-s-triazine (210.6g) was dissolved in 2L of dichloromethane, and 3.4-dimethoxyphenylacetic acid (196g) was added to In the above dichloromethane solution, stir and lower the temperature to 0-10°C, add N-methylmorpholine (121.2g) dropwise to the above system, continue stirring for 3 hours after the addition, and then analyze the raw material 3,4-dimethoxy by TLC Phenylacetic acid basically disappeared, and 2,2-dimethoxyethylamine (105 g) was added dropwise to the reaction solution. Stirring was continued for 2 h after addition, and the intermediate active ester disappeared according to TLC analysis. Suction filtration, the filtrate was successively washed with saturated NaHCO 3 solution, NaCl solution washing, anhydrous Na 2 SO 4 Dry it, filter it with suction, and evaporate it below 45°C under reduced pressure to obtain 261 g (compound IV) as a light yellow blocky solid, with a yield of 92.3%.
Embodiment 2
[0035]Dissolve N,N-dicyclohexyldiimine (250g) in 2L of dichloromethane, then add 2,2-dimethoxyethylamine (130g), and then lower the temperature to 0-20°C. Add 3.4-dimethoxyphenylacetic acid (250 g) in batches, stir and lower the temperature by 10-20° C., and stir until the raw material disappears. After suction filtration, the filter cake was washed with a small amount of dichloromethane, and then the filtrate was placed at 0-5°C for 12 hours, a little white precipitate was precipitated, filtered, and the filtrate was evaporated to dryness to obtain 206 g of (Compound IV), with a yield of 74%.
Embodiment 3
[0037] 2,4-dimethoxy-6-chloro-1,3,5-s-triazine (5.25kg) was dissolved in 30L of dichloromethane, and 3.4-dimethoxyphenylacetic acid (5kg) was added to In the above dichloromethane solution, stir and lower the temperature to 0-10°C, add N-methylmorpholine (3.0kg) dropwise to the above system, continue stirring for 3 hours after the addition, and then analyze the raw material 3,4-dimethoxy by TLC Phenylacetic acid basically disappeared, and 2,2-dimethoxyethylamine (2.6kg) was added dropwise to the reaction solution. Stirring was continued for 2 hours after the addition, and the intermediate active ester disappeared according to TLC analysis. Suction filtration, the filtrate was successively washed with saturated NaHCO 3 solution, NaCl solution washing, anhydrous Na 2 SO 4 Dry it, filter it with suction, and evaporate it below 45° C. to obtain 6.579 kg (compound IV) as a light yellow lumpy solid, with a yield of 93.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com