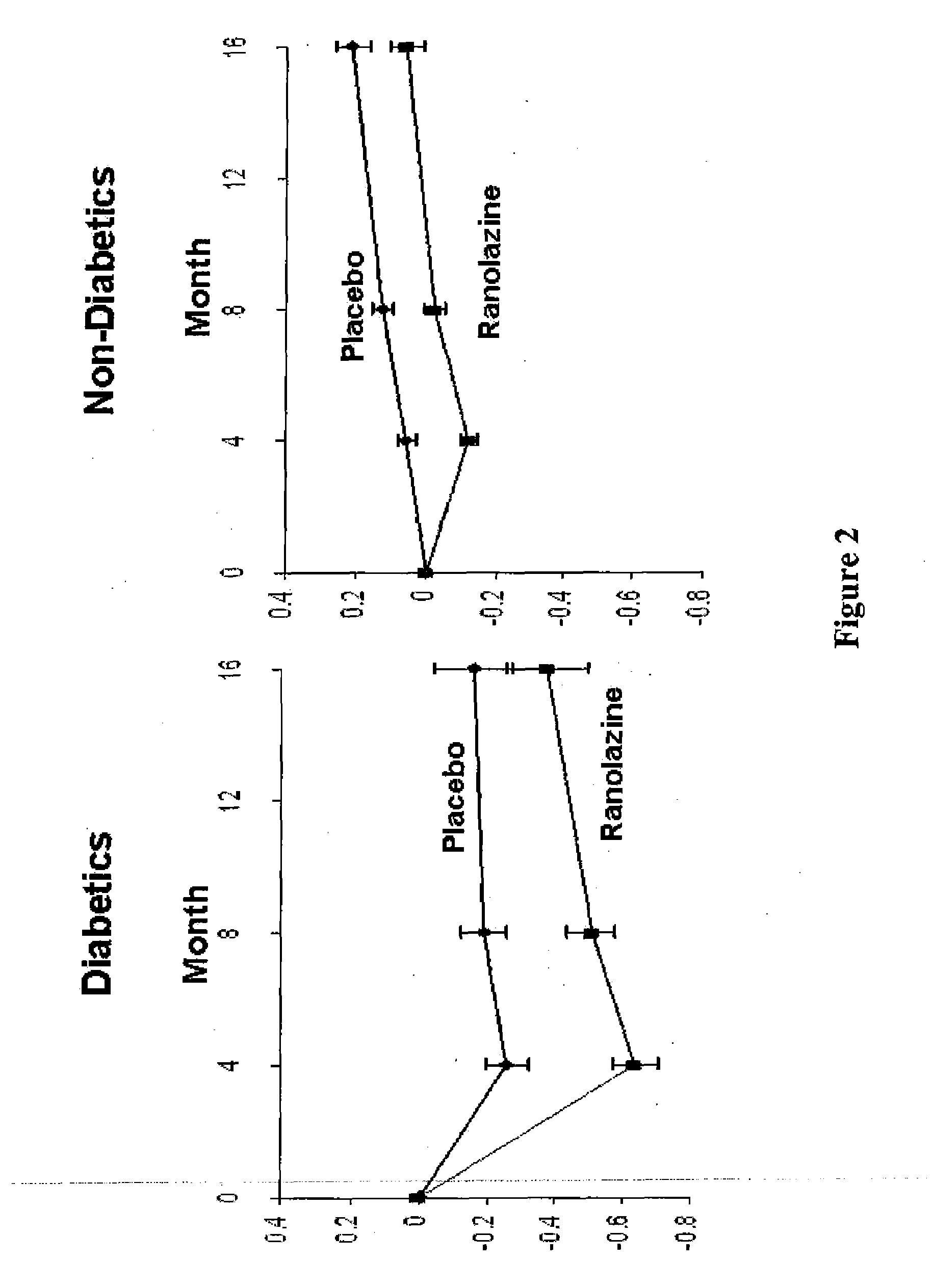
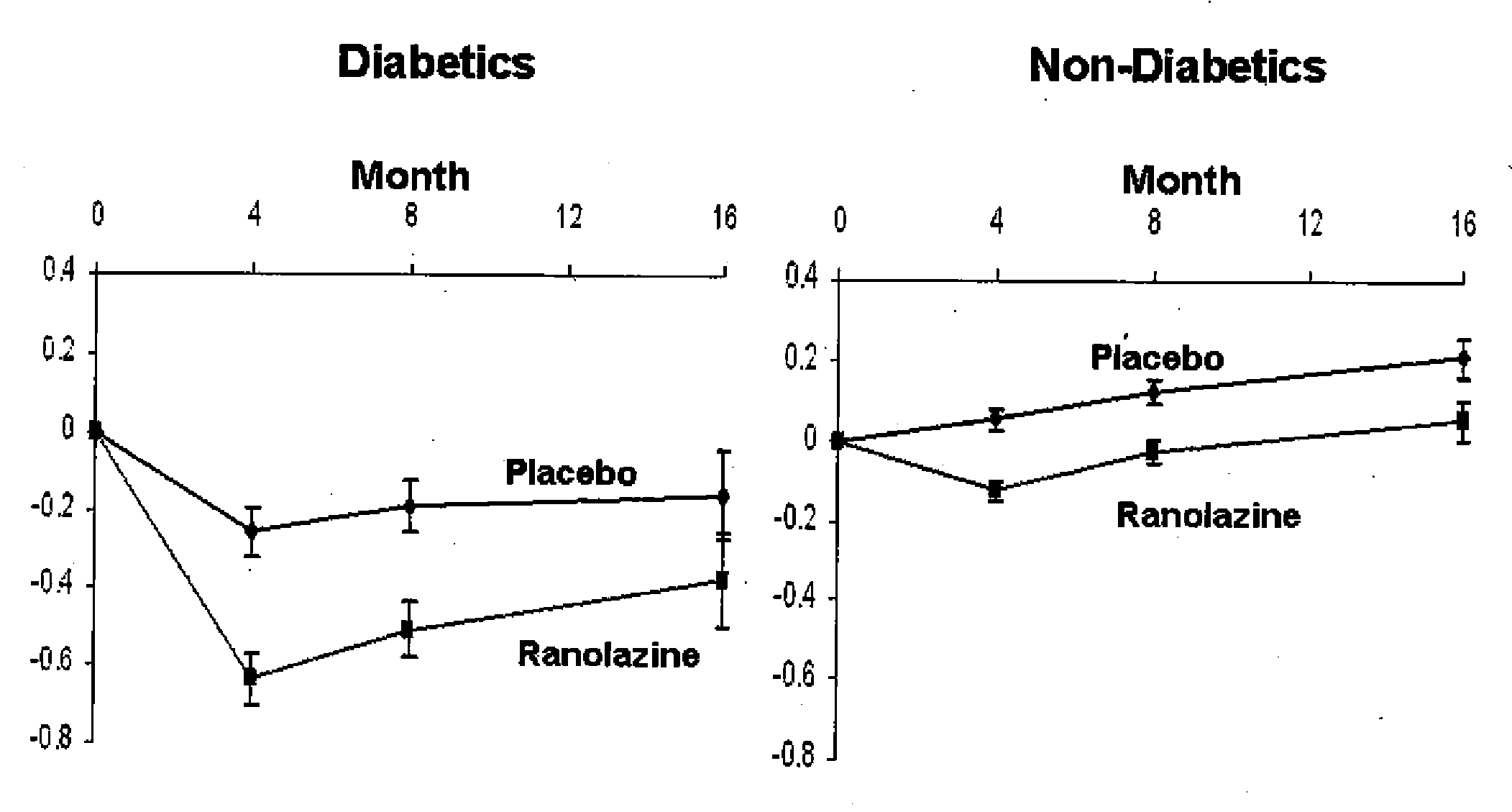
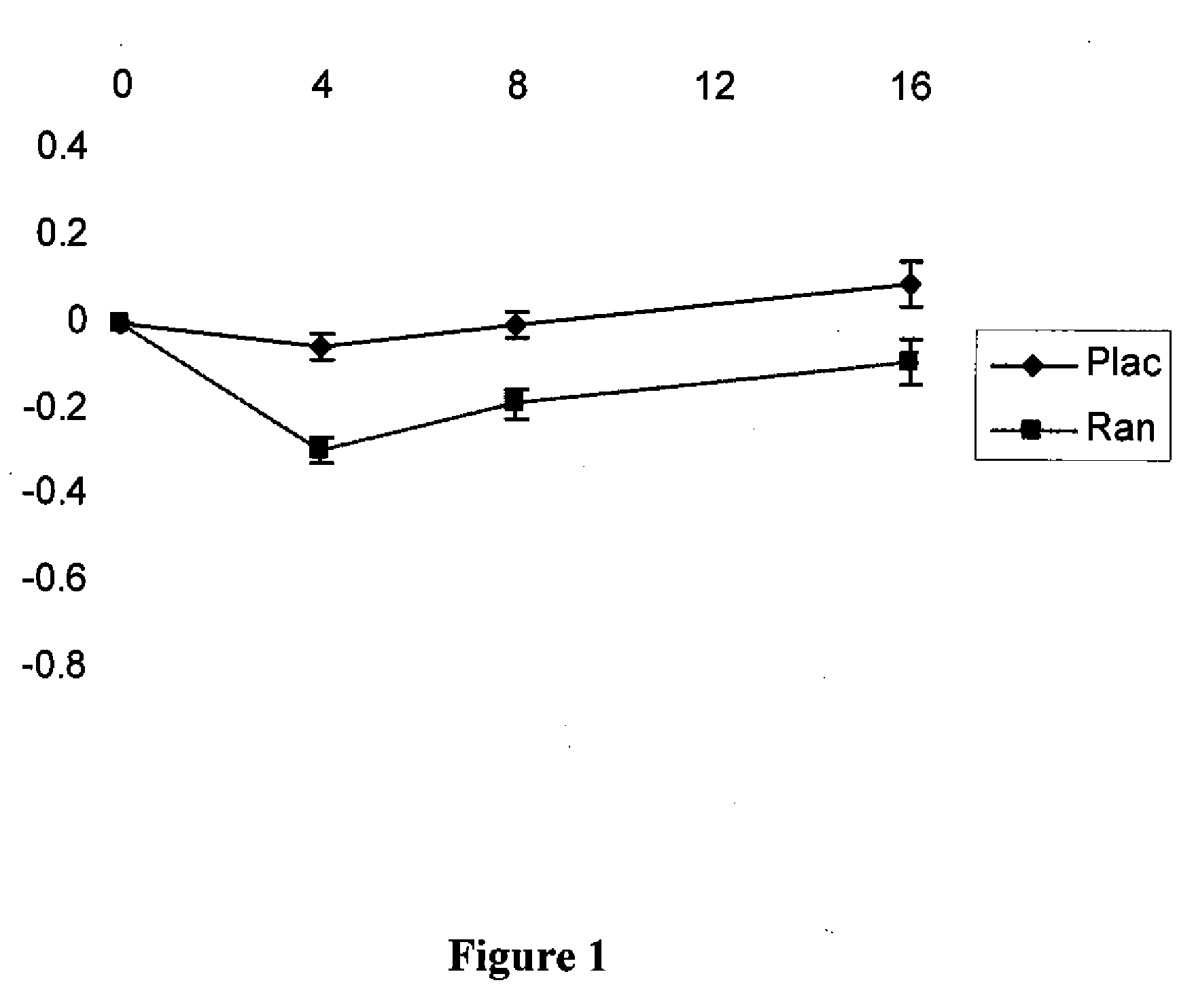
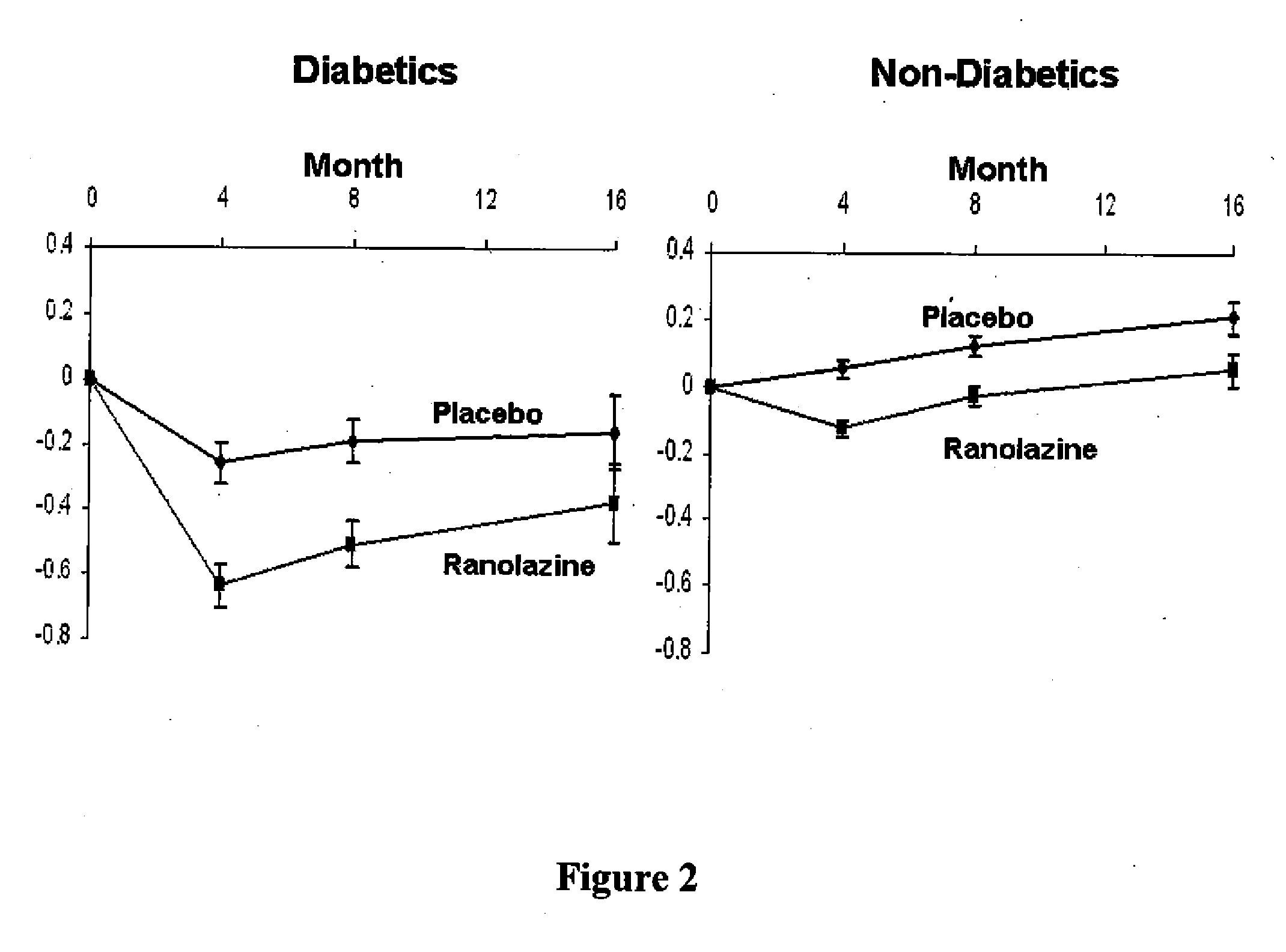
Patents
Literature
73 results about "Ranolazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
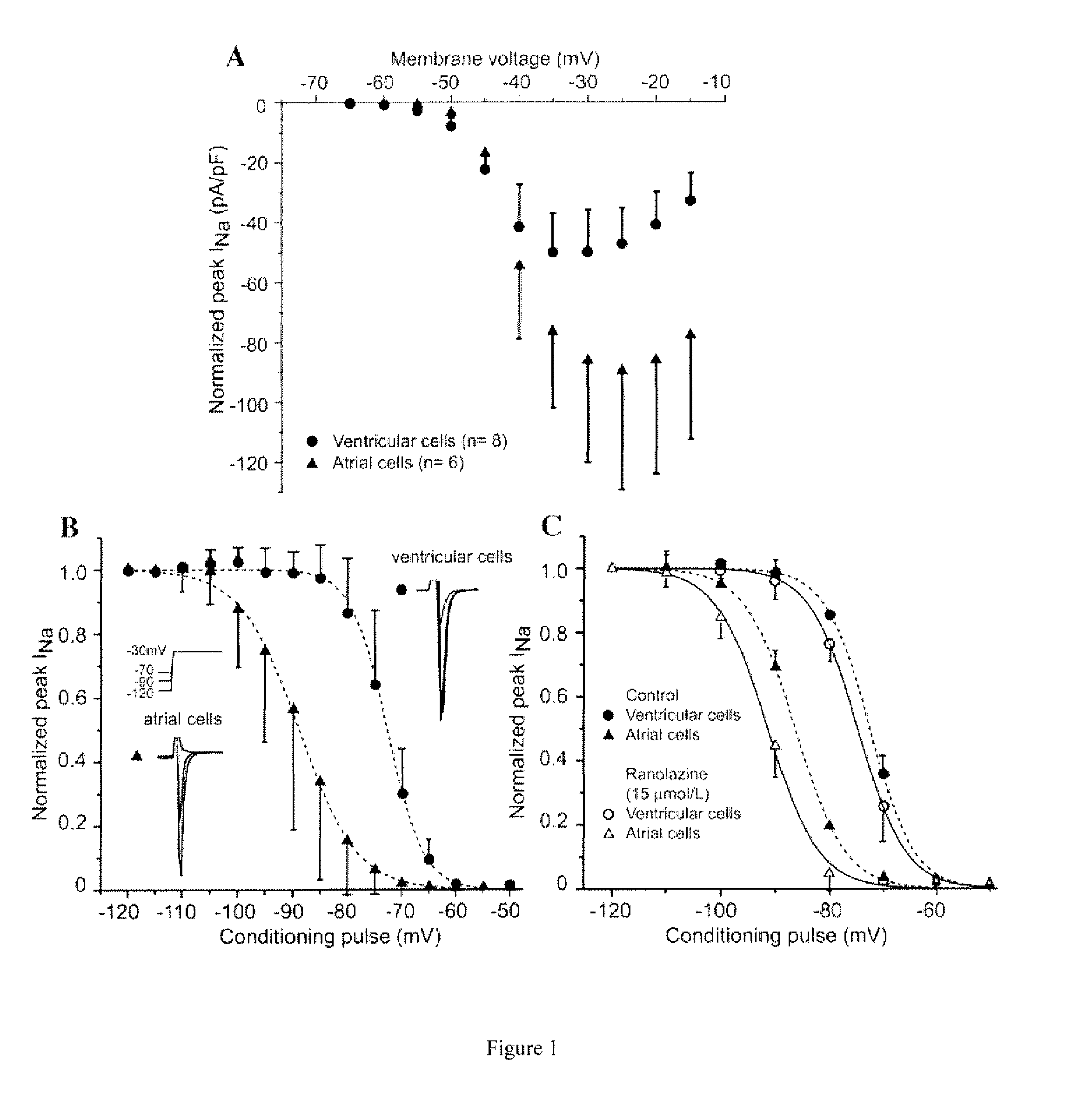
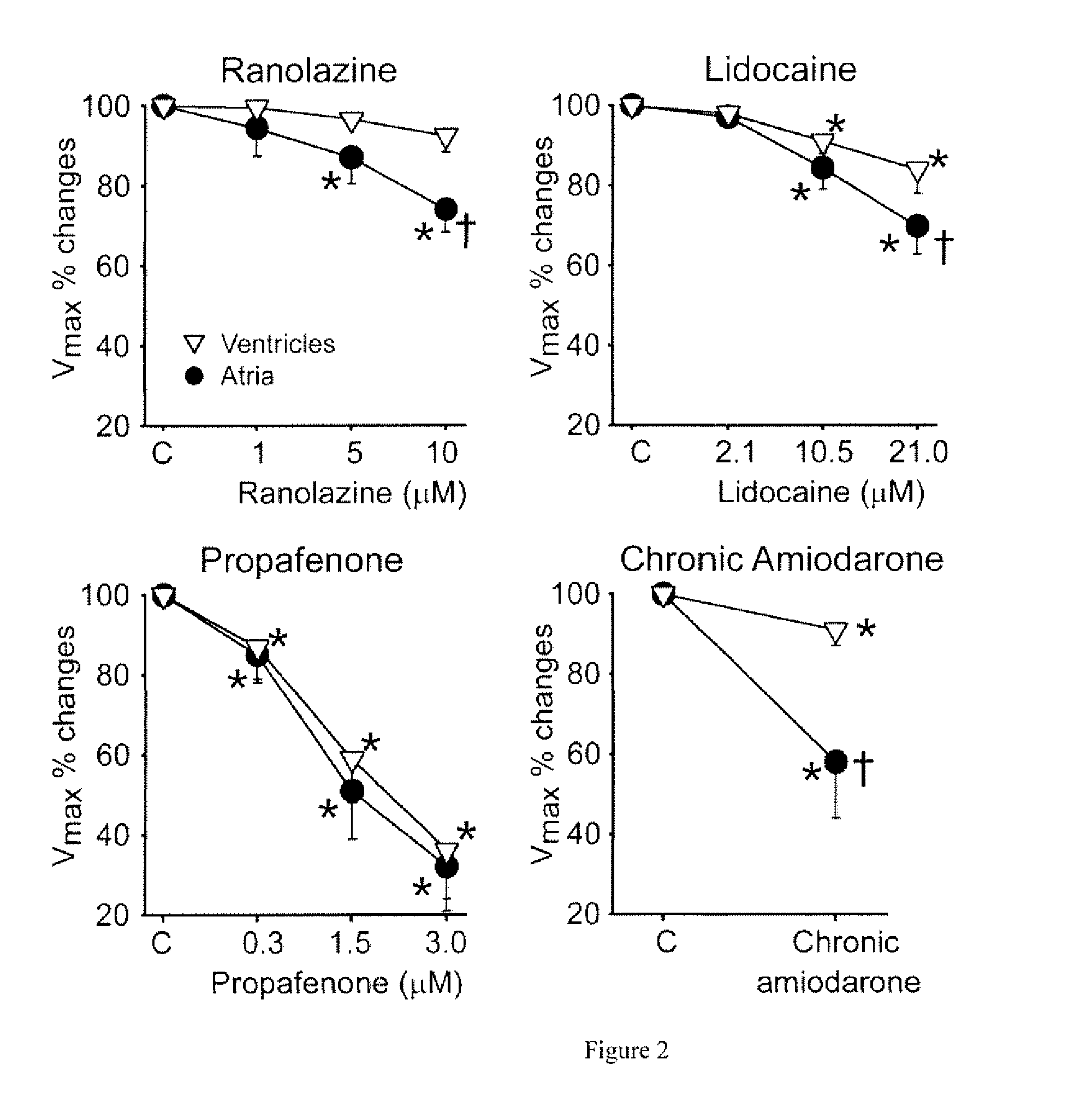
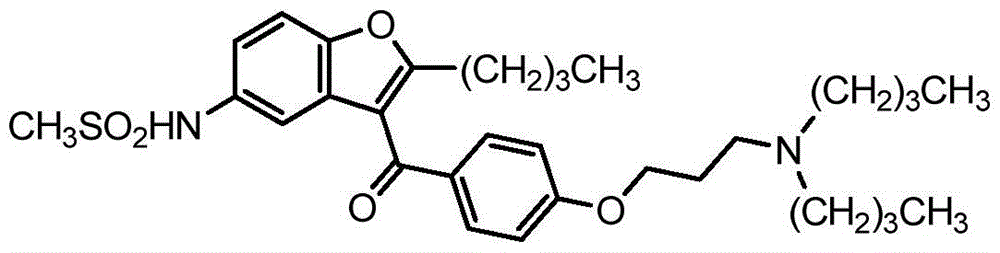
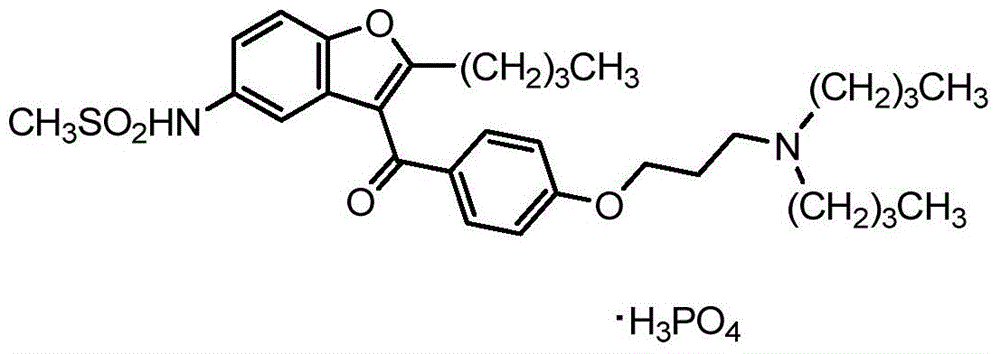
Ranolazine is used to treat a certain type of chest pain (chronic stable angina).
Methods for treating pain
InactiveUS20090203707A1Increase pulse duration of pulseImprove actionOrganic active ingredientsNervous disorderNeuropathic painRanolazine
This invention relates to methods for treating a patient suffering from neuropathic or nociceptive pain which may be mechanical, visceral, and / or inflammatory in nature, comprising administering a therapeutically effective amount of Ranolazine to a patient in need thereof.
Owner:GILEAD SCI INC
Method of treating atrial fibrillation
InactiveUS20110183990A1Reduce the amplitudeReduce adverse side effectsBiocideAnimal repellantsDronedaroneRanolazine
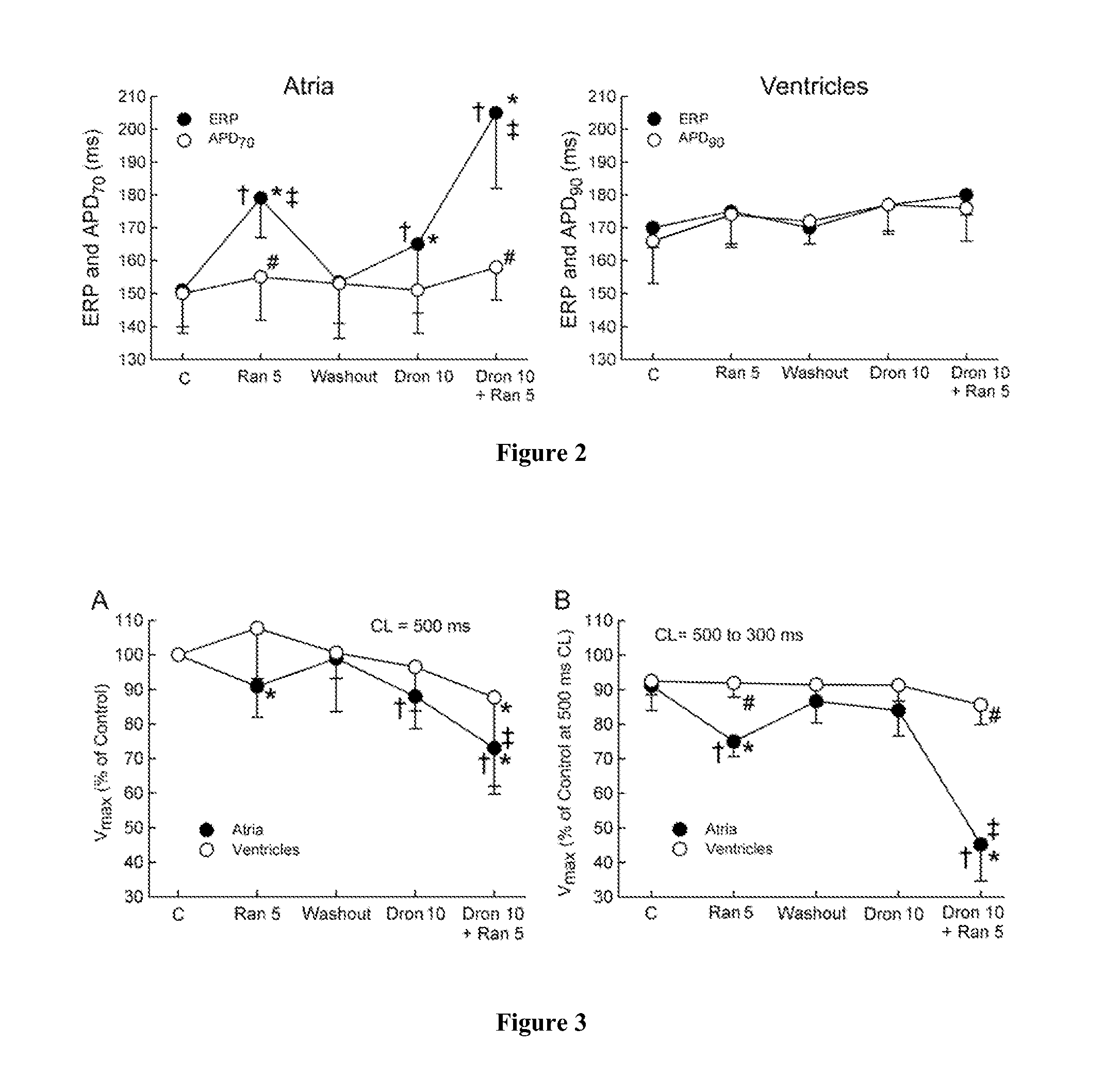
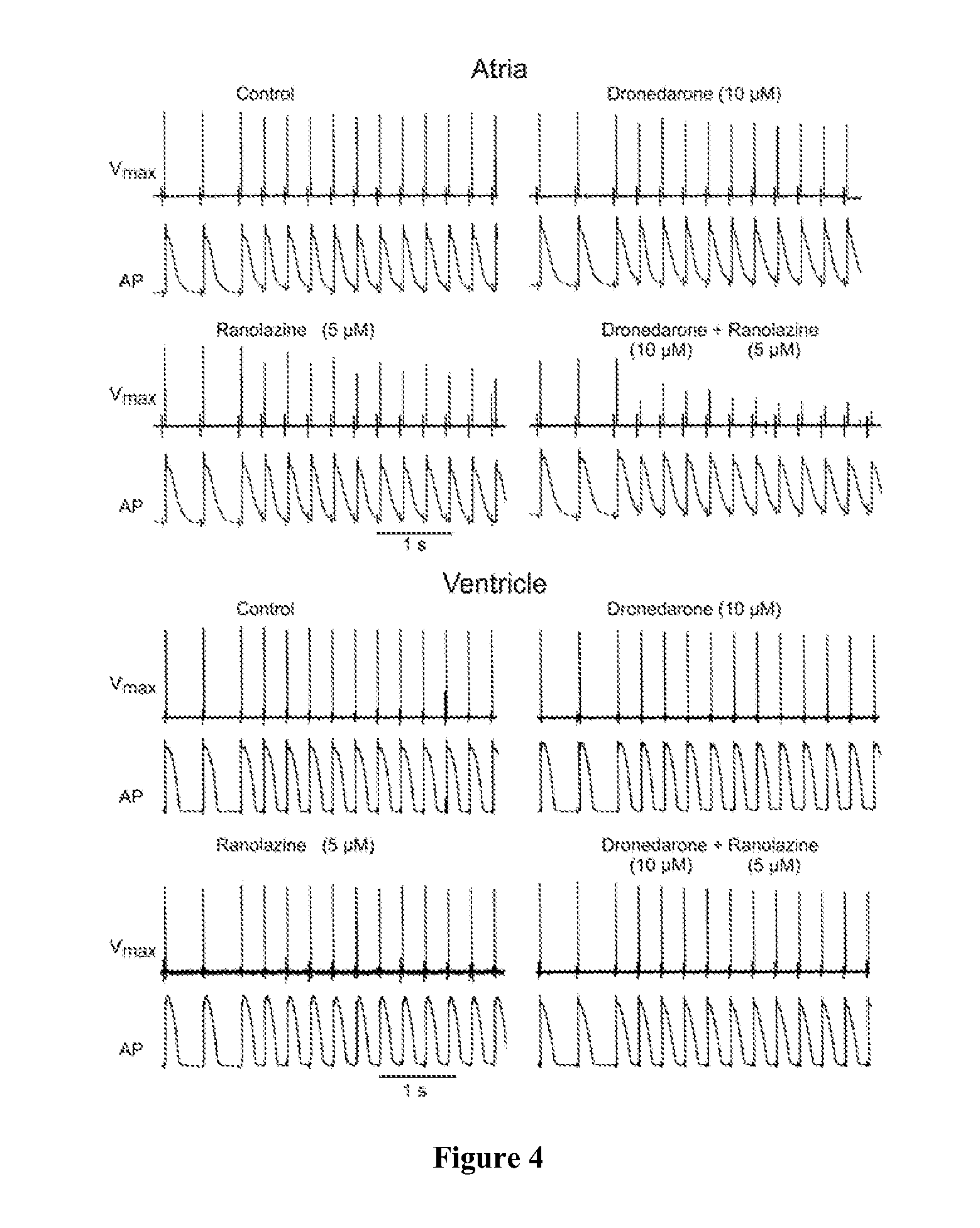
The present invention relates to a method for the treatment or prevention of atrial fibrillation and / or atrial flutter comprising coadministration of a synergistically therapeutic amount of dronedarone or a pharmaceutically acceptable salt or salts thereof and a synergistically therapeutic amount of ranolazine or a pharmaceutically acceptable salt or salts thereof. Also provided are methods for modulating ventricular and atrial rhythm and rate. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
Method of treating atrial fibrillation
InactiveUS20100056536A1Prolongs repolarizationPositive take-off potentialOrganic active ingredientsCardiovascular disorderRanolazineAmiodarone
The present invention relates to a method for the treatment of atrial fibrillation comprising the coadministration of a synergistic therapeutically effective amount of amiodarone and synergistic therapeutically effective amount ranolazine. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
Sustained release ranolazine formulations
InactiveUS6852724B2Without fluctuationBiocidePharmaceutical non-active ingredientsRanolazineDissolution
A sustained release ranolazine formulation contains an intimate mixture of ranolazine and a partially neutralized pH-dependent binder to form a film that is mostly insoluble in aqueous media below pH 4.5 and soluble in aqueous media above pH 4.5. The formulation is suitable for twice daily administration of ranolazine and is useful for controlling the rate of dissolution of ranolazine, and to maintain human plasma ranolazine levels at between 850 and 4000 ng base / mL.
Owner:GILEAD SCI INC
Sustained release pharmaceutical formulations
Owner:GILEAD SCI INC
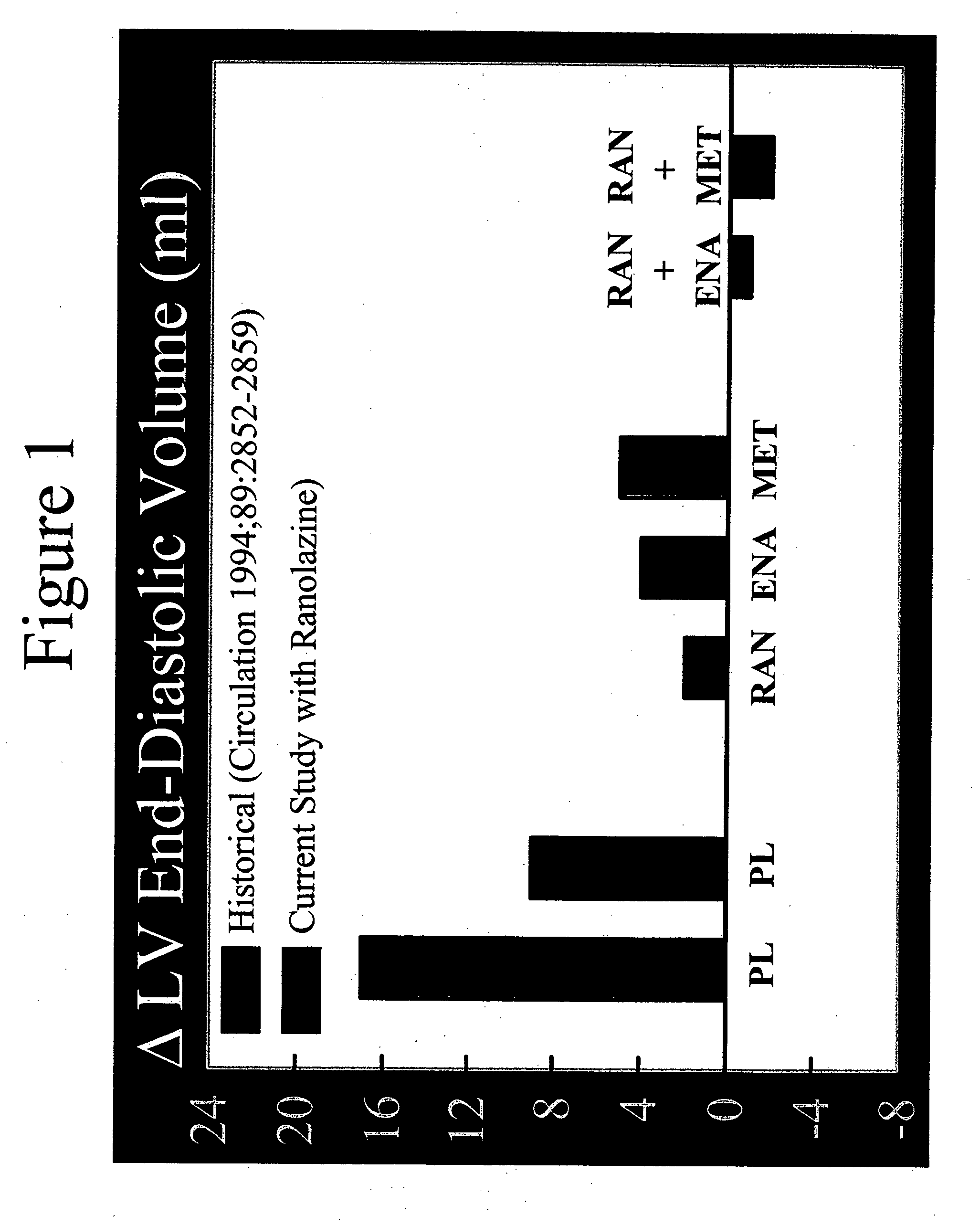
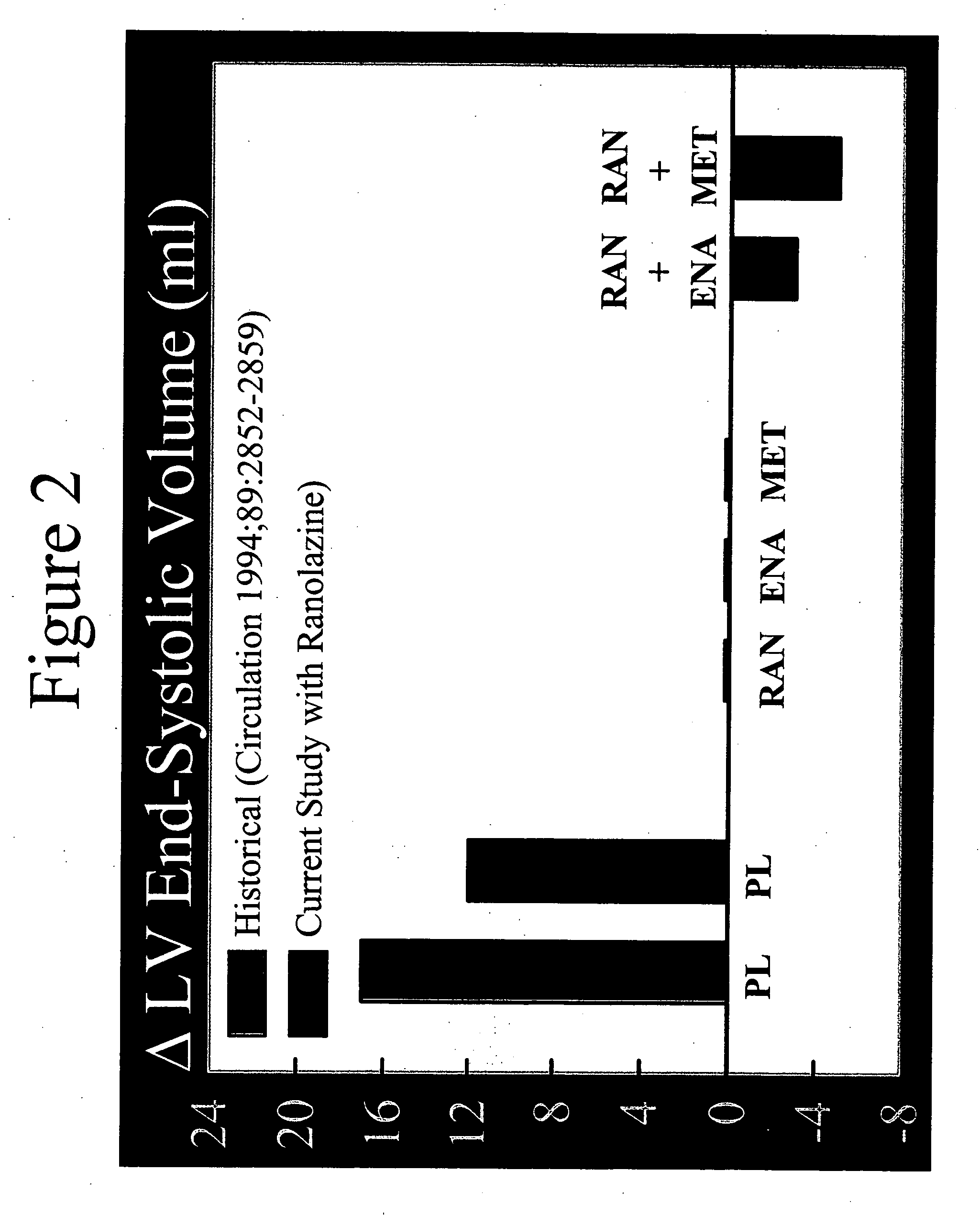
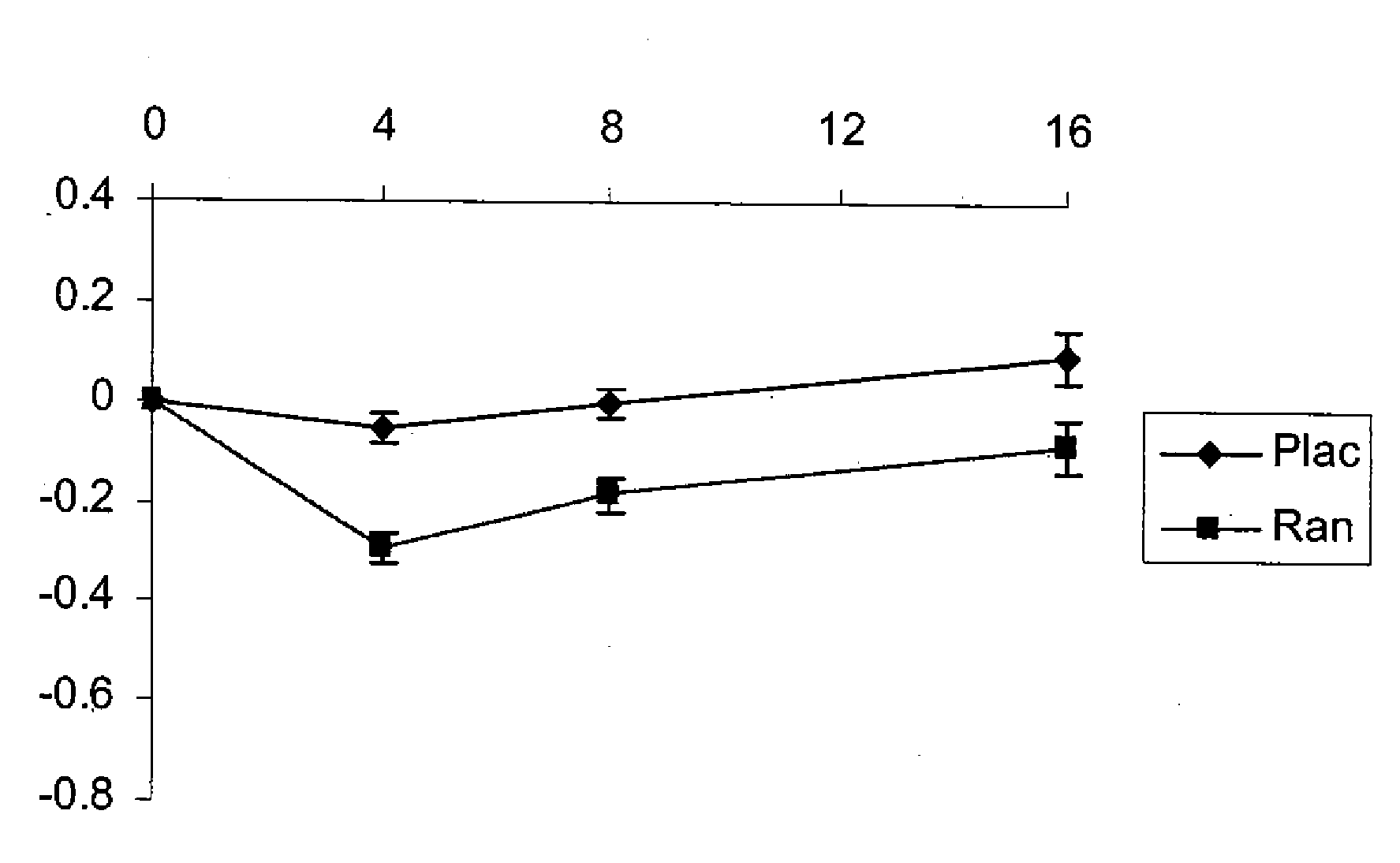
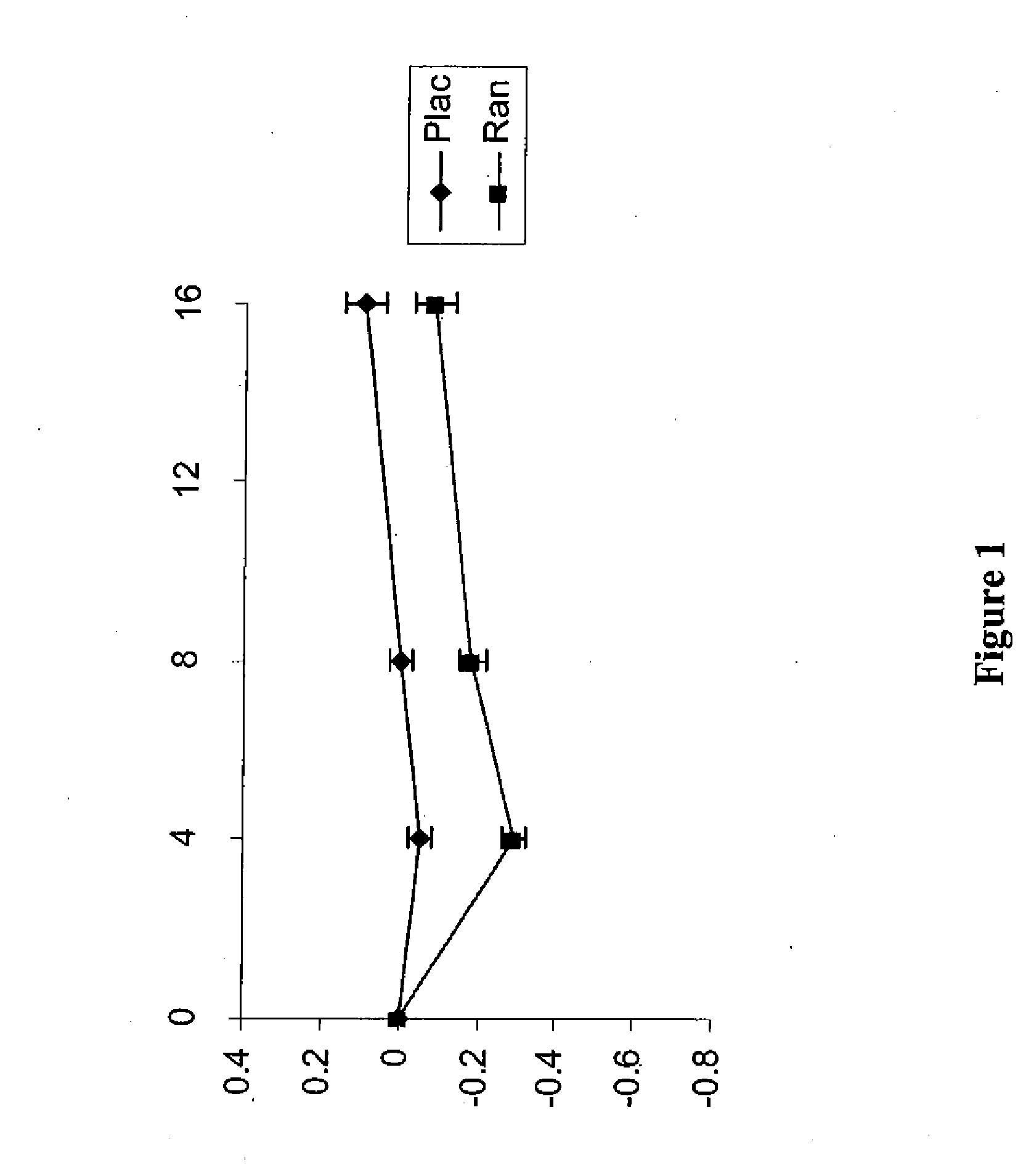
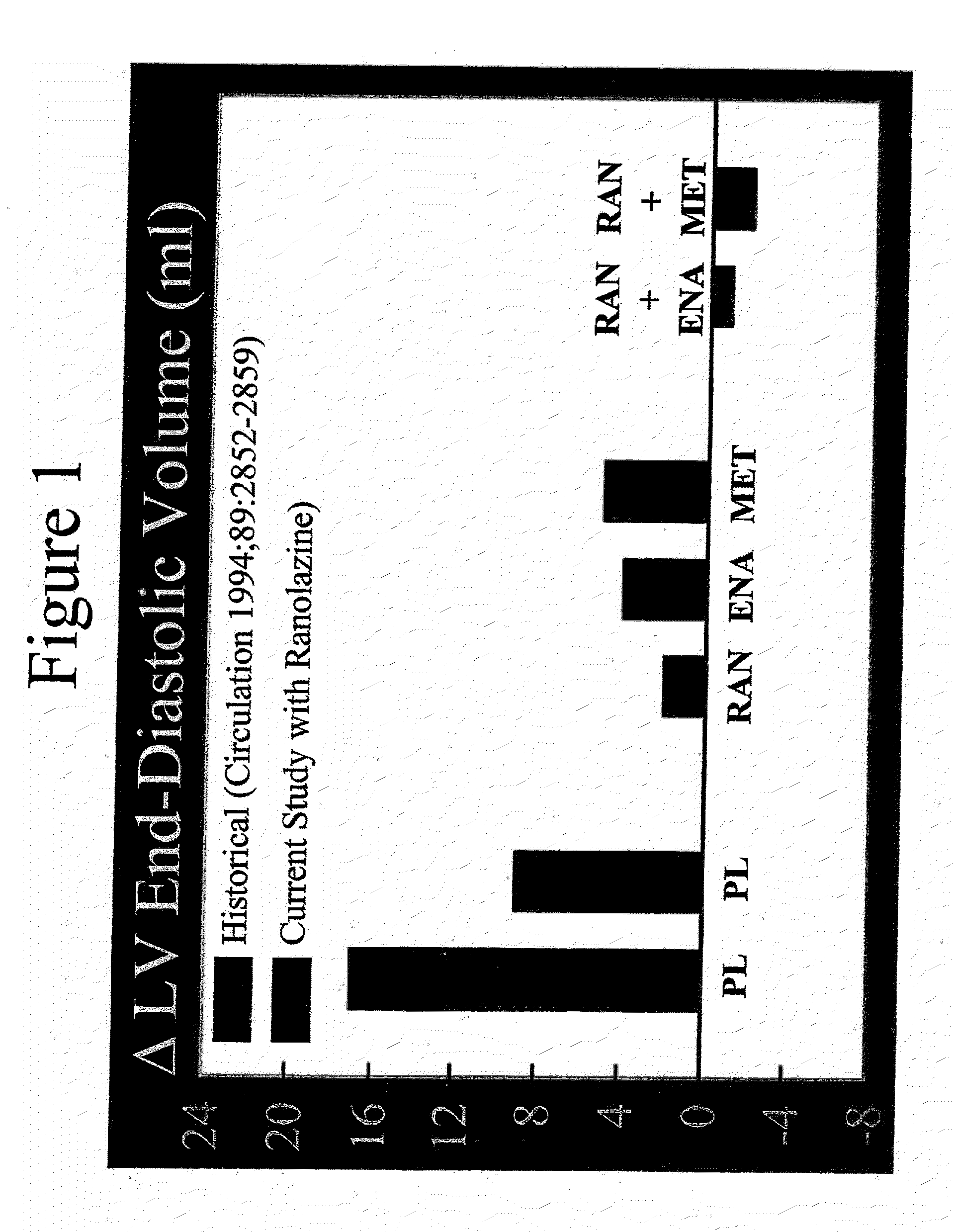
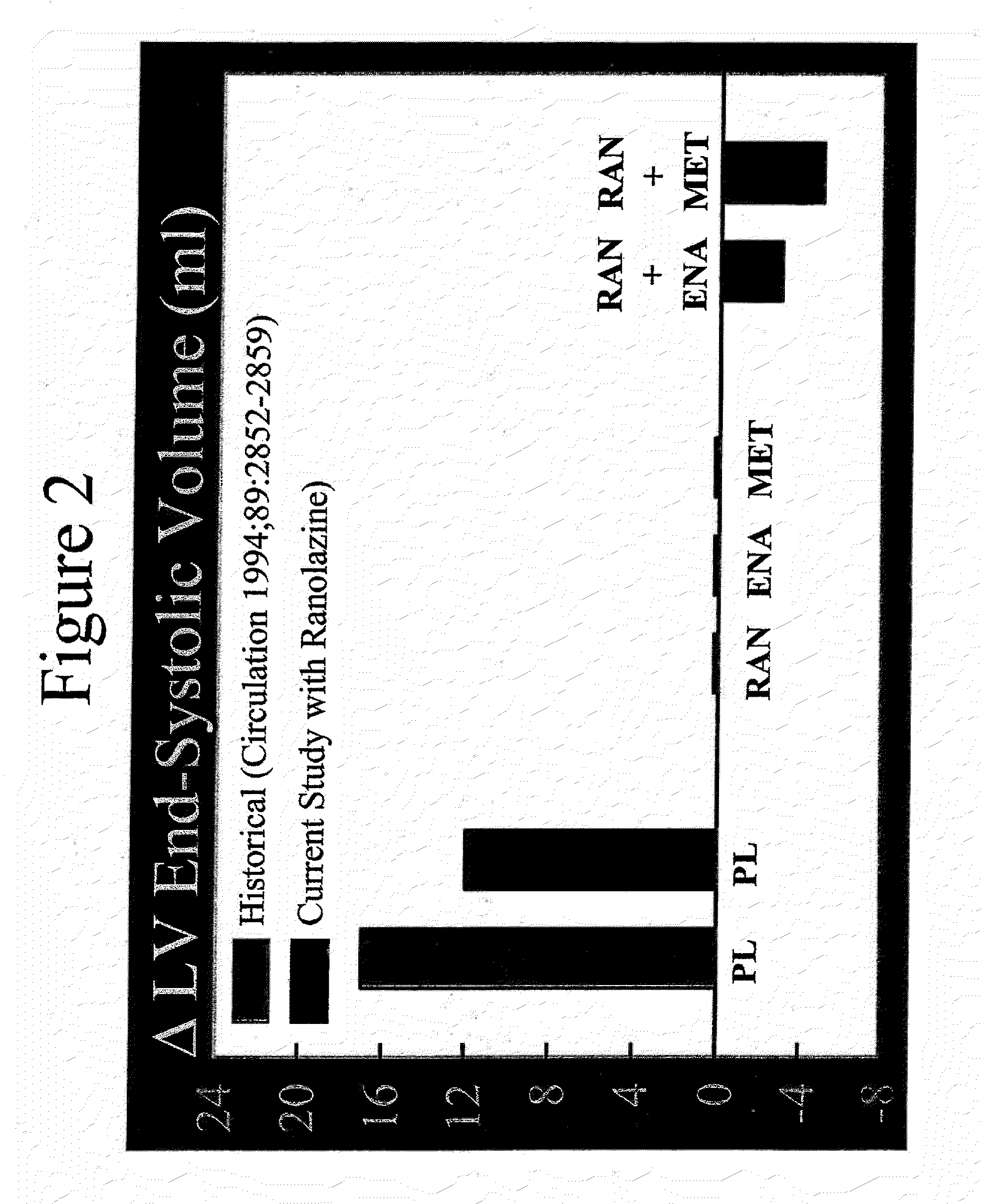
Method of reversing left ventricle remodeling
InactiveUS20060111361A1Reduction in LV volumeCardiac outputBiocideAnimal repellantsBeta blockerRanolazine
The present invention relates to method of reversing left ventricle remodeling by combined administration of therapeutically effective amounts ranolazine and at least one co-remodeling agent, which may be an ACE inhibitor, an ARB, or a beta-blocker. The method finds utility in the treatment of heart failure. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
Use of ranolazine for the treatment of coronary microvascular diseases
Disclosed are methods for treating patients suffering from coronary microvascular disease comprising administering ranolazine to the patient. In one embodiment, ranolazine is administered as an oral dose
Owner:GILEAD SCI INC
Use of ranolazine for the treatment of non-coronary microvascular diseases
Disclosed are methods for treating patients suffering from non-coronary microvascular disease comprising administering ranolazine to the patient. In one embodiment, ranolazine is administered as an oral dose.
Owner:GILEAD SCI INC
Method of reversing left ventricular remodeling
InactiveUS20090176772A1Improve actionReduce releaseCardiovascular disorderHeterocyclic compound active ingredientsBeta blockerLeft ventricular size
The present invention relates to method of reversing left ventricle remodeling by combined administration of therapeutically effective amounts ranolazine and at least one co-remodeling agent, which may be an ACE inhibitor, an ARB, or a beta-blocker. The method finds utility in the treatment of heart failure. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
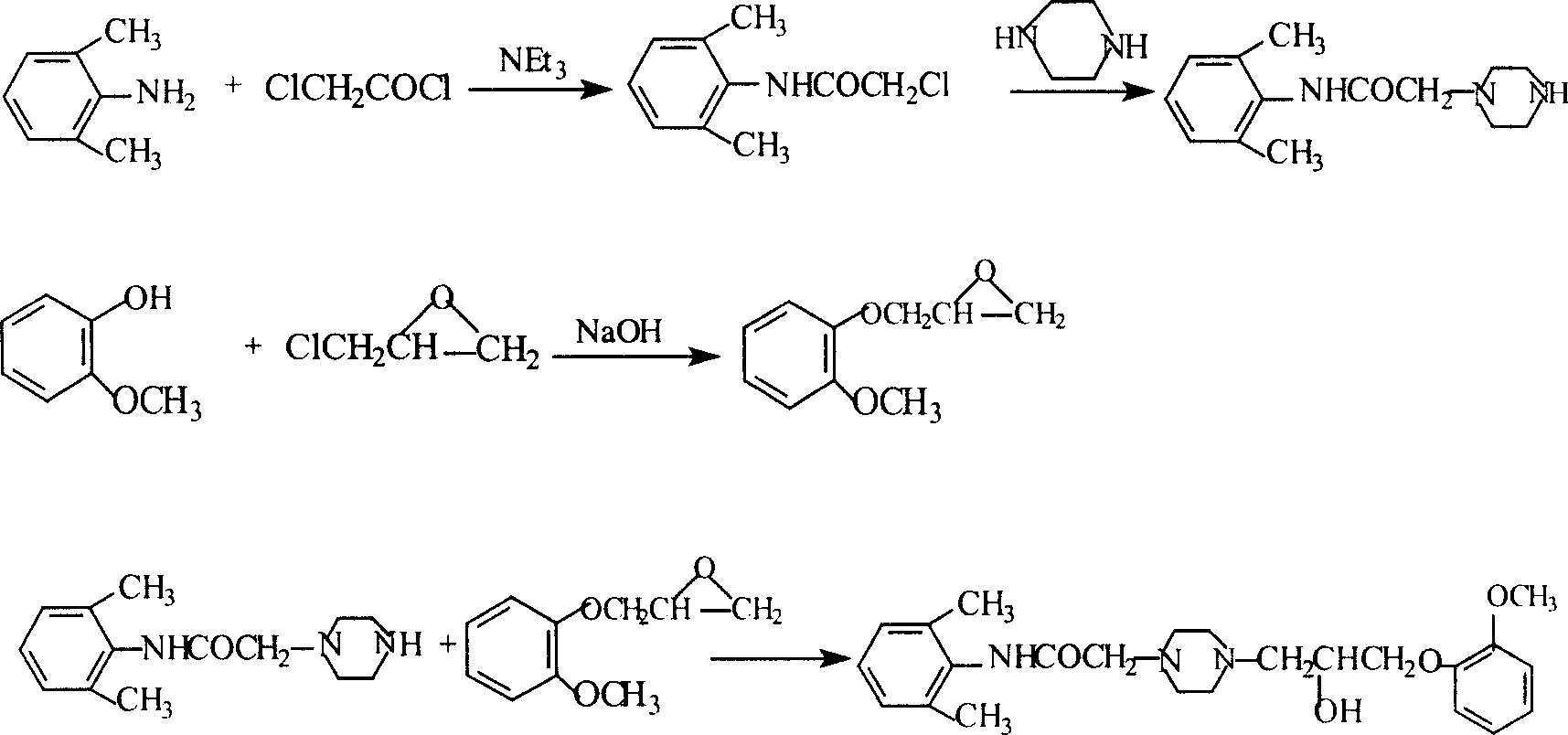
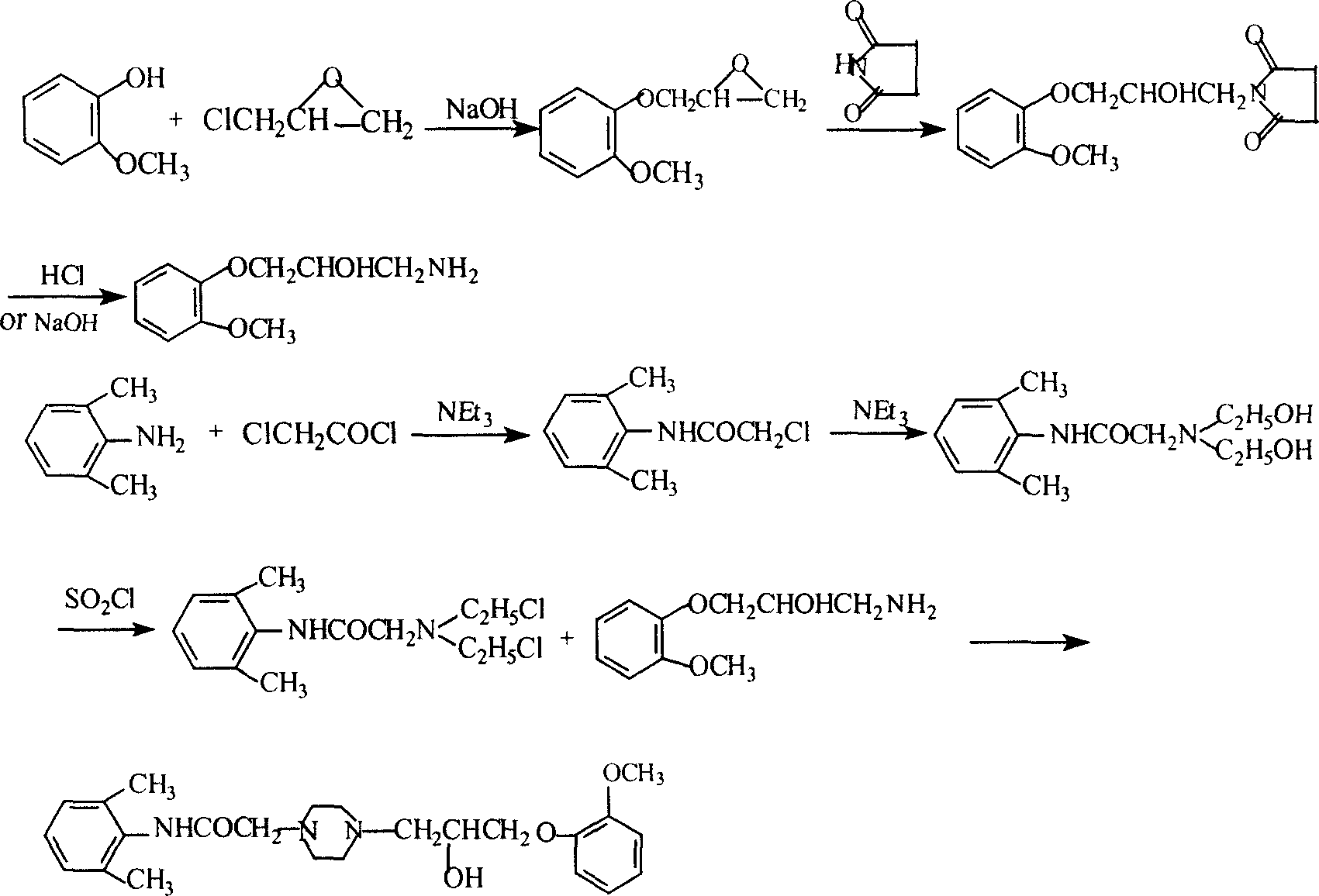
Method for synthesizing Ranolazine
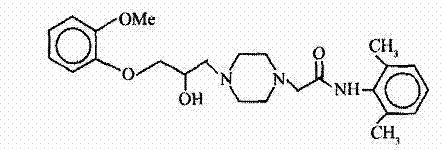
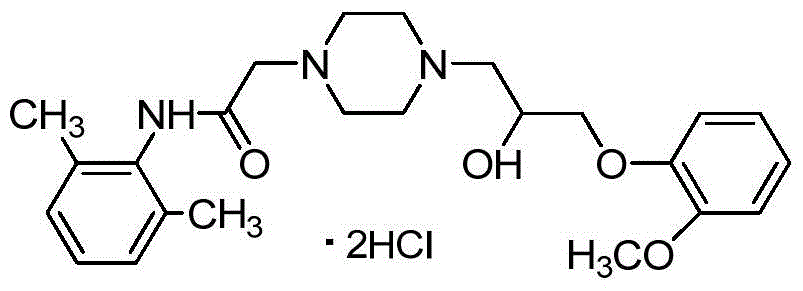
This invention relates to a method for synthesizing ranolazine drug for treating stenocardia. The method comprises: performing amidation and N-monoalkylation on 2, 6-dimethylaniline to obtain N-(2, 6-xylyl)-2-(1-piperazine) acetamide, and then reacting with 2-(2-methoxyphenoxy) epoxyethane generated from o-methoxyphenol and epoxy chloropropane to obtain anolazine. The reactions include the refinery of 2-chloro-N-(2,6-xylyl)acetamide by cyclohexane and the recrystallization of ranolazine by ethanol / ethyl acetate (2:1), thus can raise the yield.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Improved method for synthesizing ranolazine
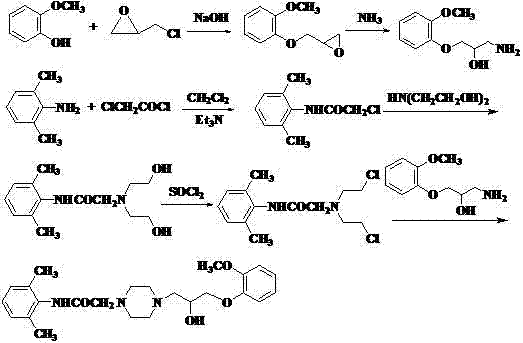
ActiveCN102558097AReflux reaction time shortenedShort reaction timeOrganic chemistryDimethylaniline N-oxideDimethylphenylpiperazinium
The invention discloses an improved method for synthesizing an antianginal medicine ranolazine and belongs to the field of pharmaceutical chemistry. The method comprises the following steps of: amidating 2,6-dimethylaniline which is taken as a raw material, and alkylating to obtain N-(2,6-dimethylphenyl)-2-(1-piperazino)-acetamide; and reacting the N-(2,6-dimethylphenyl)-2-(1-piperazino)-acetamide and 2-(2-methylphenoxymethyl)ethylene oxide which is obtained by reaction of 2-methoxyphenol and epichlorohydrin to obtain crude ranolazine, and recrystallizing to obtain refined ranolazine. A reaction solvent, the molar ratio of raw materials, a phase transfer catalyst, a recrystallization solvent and the like are optimized; and the improved method is convenient to operate and is suitable for industrialized production, the production cost is reduced, and the yield is improved.
Owner:FUREN PHARMA GROUP +1
High-purity ranolazine and preparation method thereof
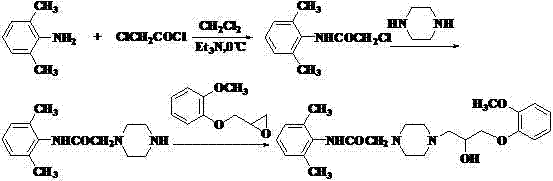
InactiveCN101560196AGood lookingHigh purityOrganic chemistryCardiovascular disorderAlkyl transferDimethylaniline N-oxide
The invention discloses a preparation technology and a refining method of ranolazine, which are more suitable for industrial production. The method comprises the following concrete steps: using 2, 6-dimethylaniline and methylpyrocatechine as original materials; sequentially carrying out four reaction processes of N-acylation, O-alkylation, N-alkylation and N-alkylation to synthesize ranolazine; and then, recrystallizing to obtain a refined product. The refined product obtained by the method has high purity, and the method also has the advantages of simple operation, low production cost and high yield and is more suitable for industrial production.
Owner:BEIJING WANQUAN SUNSHINE MEDICAL TECH CO LTD
Preparation of ranolazine
InactiveUS20110151258A1Organic chemistrySynthetic resin layered productsRanolazineMedicinal chemistry
Preparation of ranolazine and intermediates thereof, for use in pharmaceutical compositions comprising ranolazine.
Owner:DR REDDYS LAB LTD
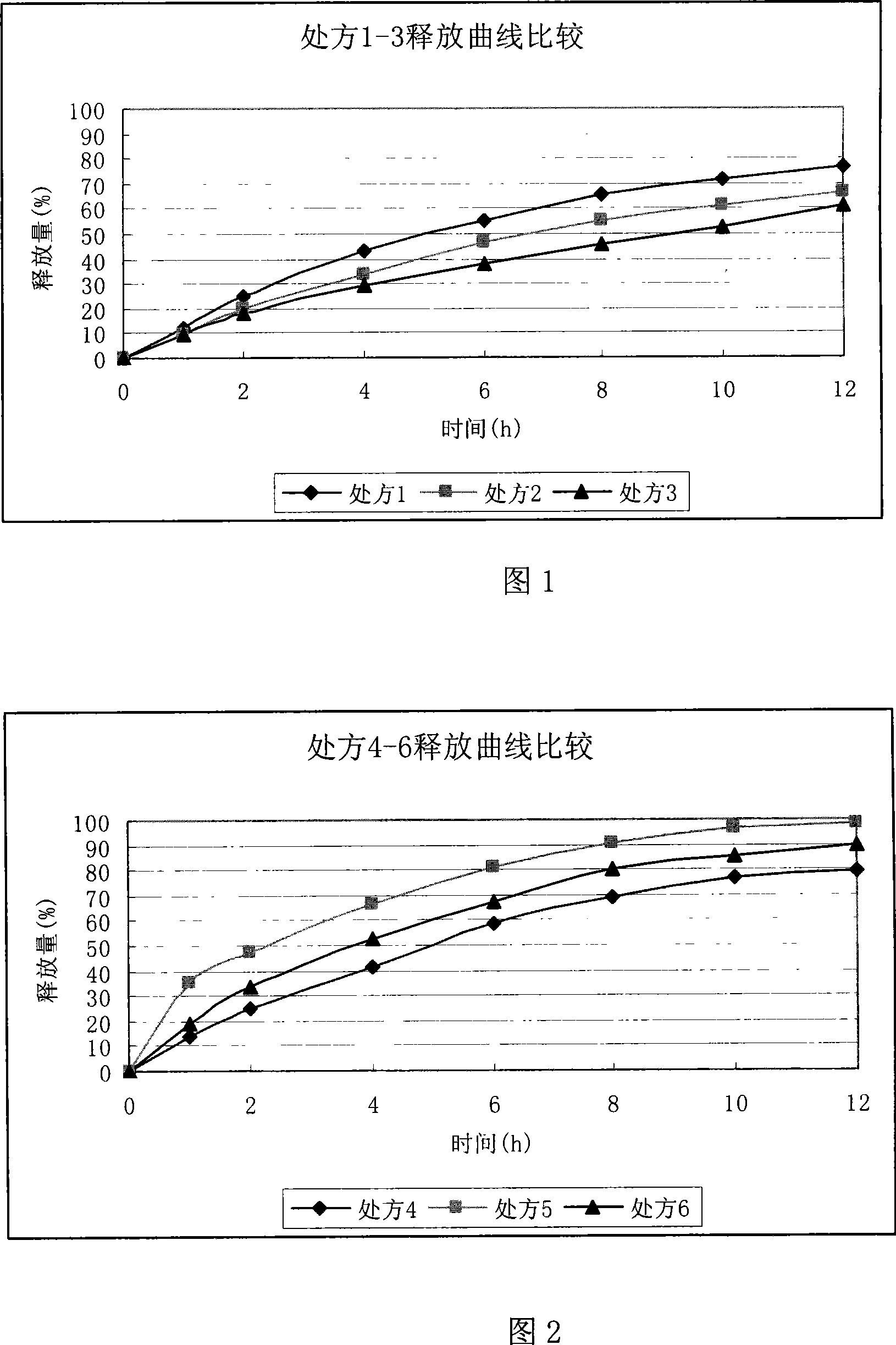
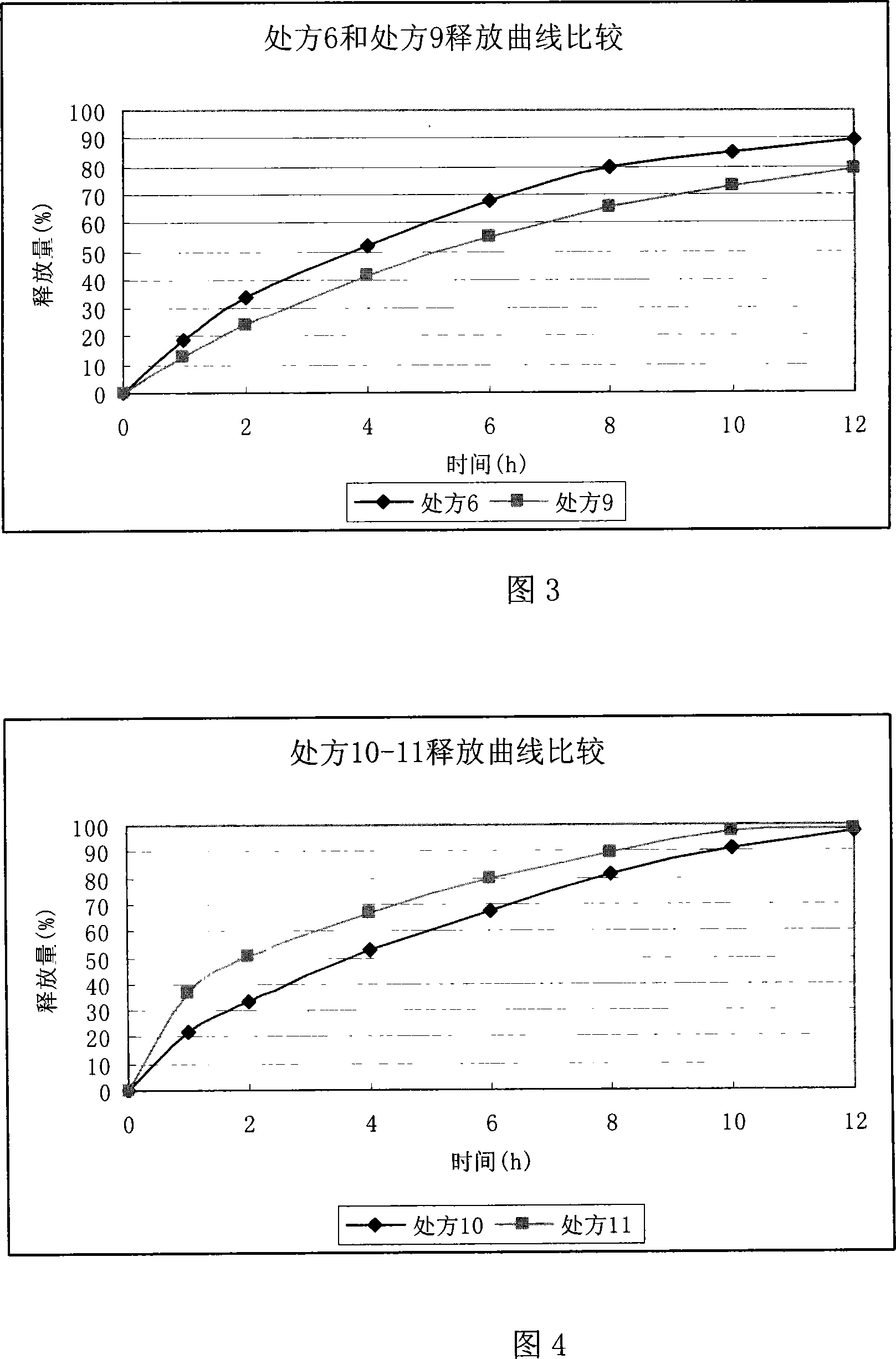
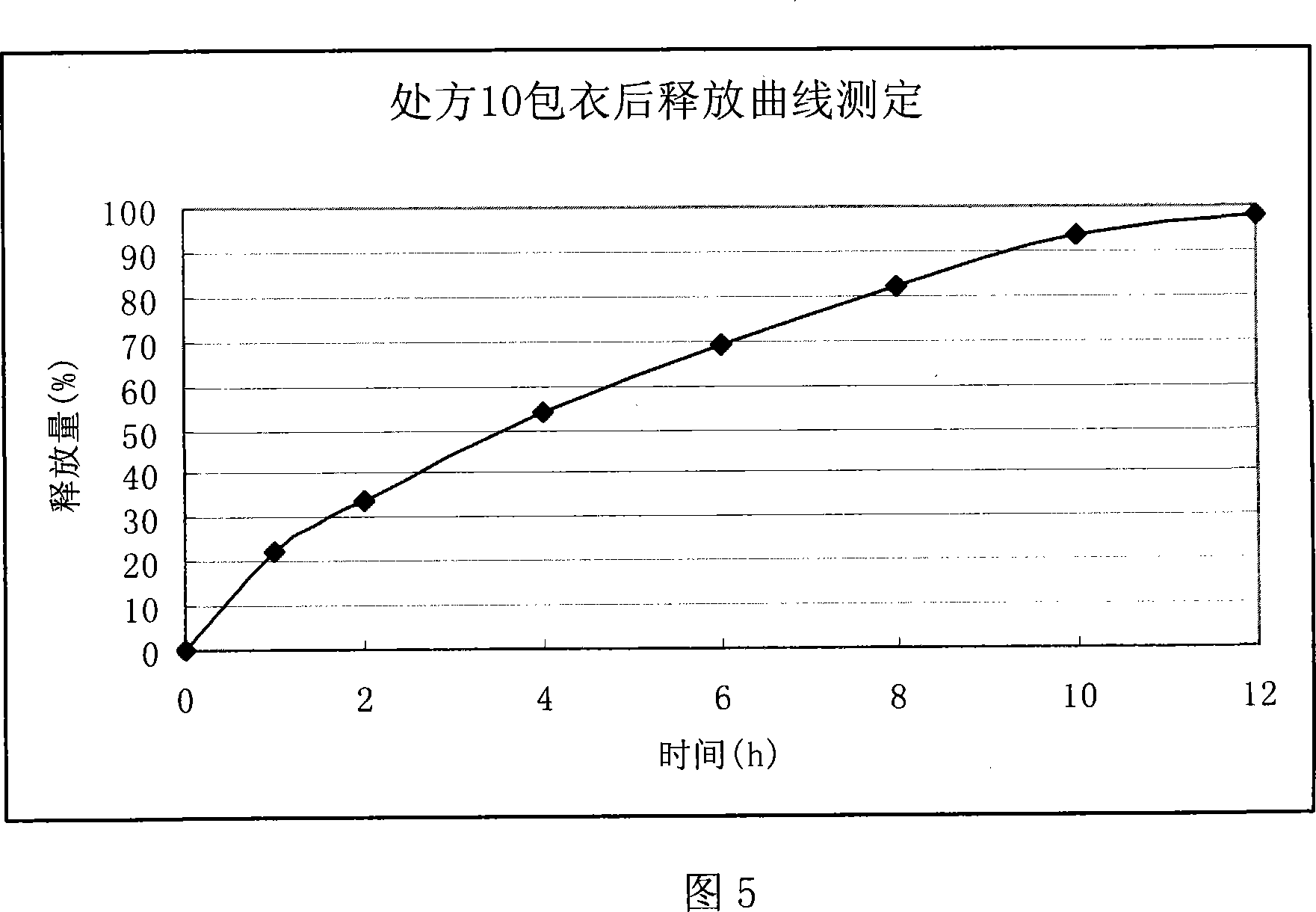
Ranolazine oral sustained-release preparation and preparation method thereof
The invention provides a Ranolazine oral sustained-release preparation and a preparation method. The Ranolazine oral sustained-release preparation comprises Ranolazine, a sustained-release skeleton material, a filling agent, an adhesive and a lubricating agent, and is characterized in that the weight of the Ranolazine is between 35 and 85 percent in the sustained-release preparation; a prescription composition is preferably selected according to a large number of experiments; hydroxypropyl methyl cellulose phthalate and methyl cellulose are served as the sustained-release skeleton material ofthe sustained-release preparation in the preferable weight ratio of 2:1-1.5; and microcrystalline cellulose is served as the filling agent; the 29 / 39 ethanol solution of polyvinyl pyrrolidone K is served as the adhesive, and magnesium stearate is served as the lubricating agent.
Owner:FUREN PHARMA GROUP
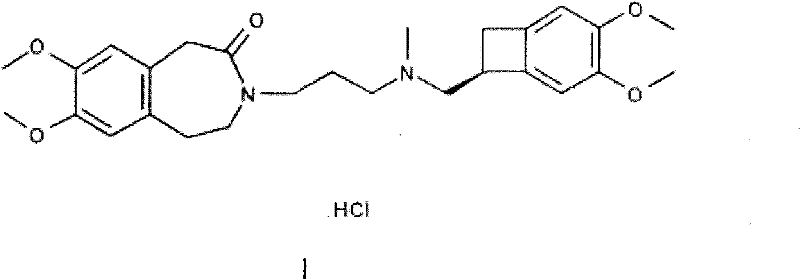
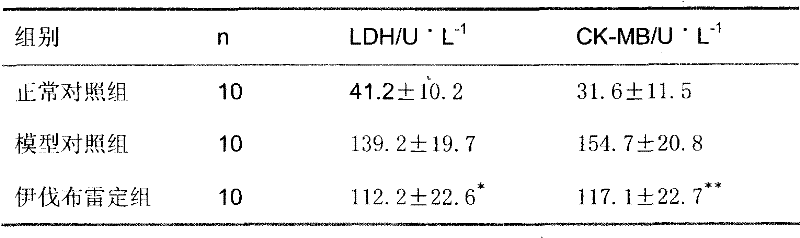
Pharmaceutical composition containing ivabradine and ranolazine
ActiveCN101780091BHazard mitigationGood synergyCardiovascular disorderHeterocyclic compound active ingredientsMedicineAngina
The present invention provides a pharmaceutical composition containing active ingredients ivabradine or a pharmacologically acceptable salt thereof and ranolazine or a pharmacologically acceptable salt thereof. Through research, the present invention finds that the combined use of ivabradine and ranolazine effectively alleviates myocardial ischemia, and shows a good synergistic effect on myocardial ischemia diseases such as angina pectoris and coronary heart disease. Therefore, a method for treating myocardial ischemic diseases with better effect and lower adverse reactions has been found, and a good solution has been found for the unsatisfactory treatment effect of myocardial ischemic diseases in clinical practice.
Owner:LUNAN PHARMA GROUP CORPORATION
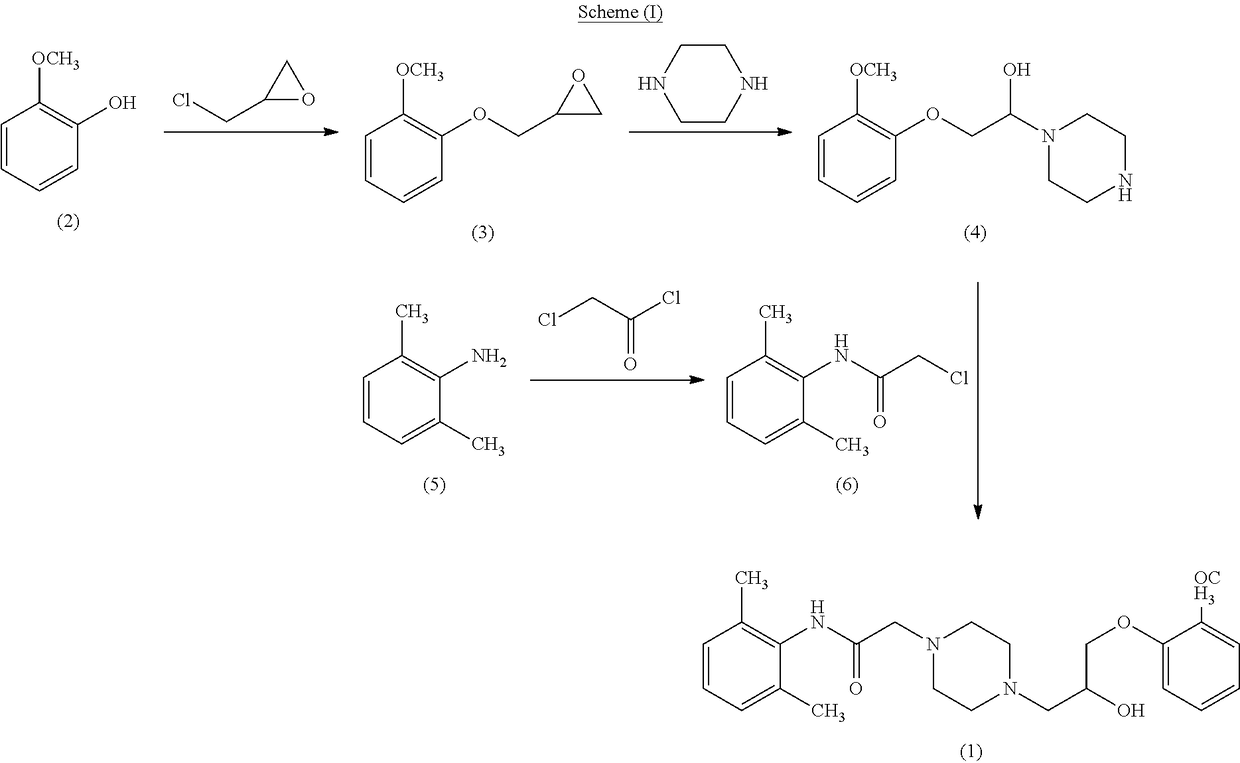
Novel process for the preparation of ranolazine
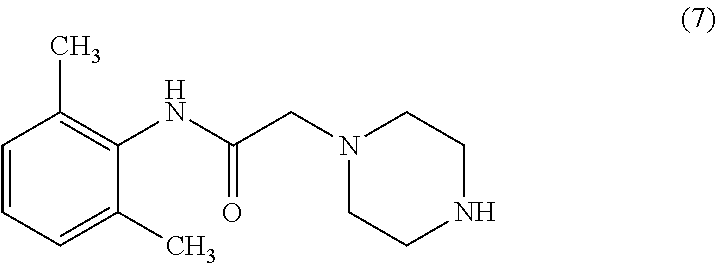
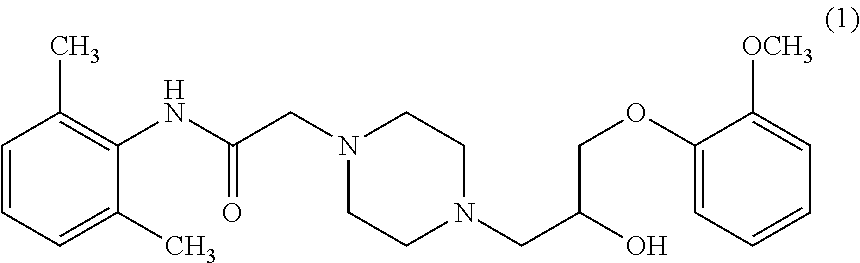
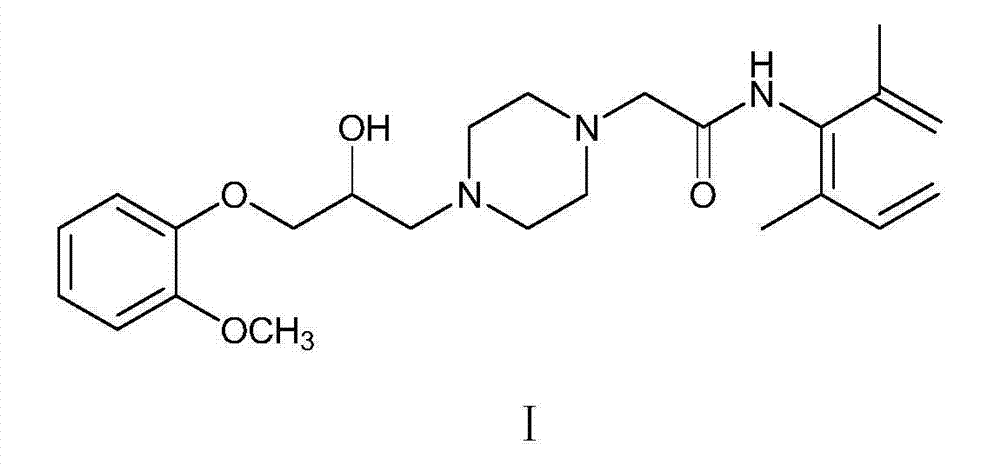
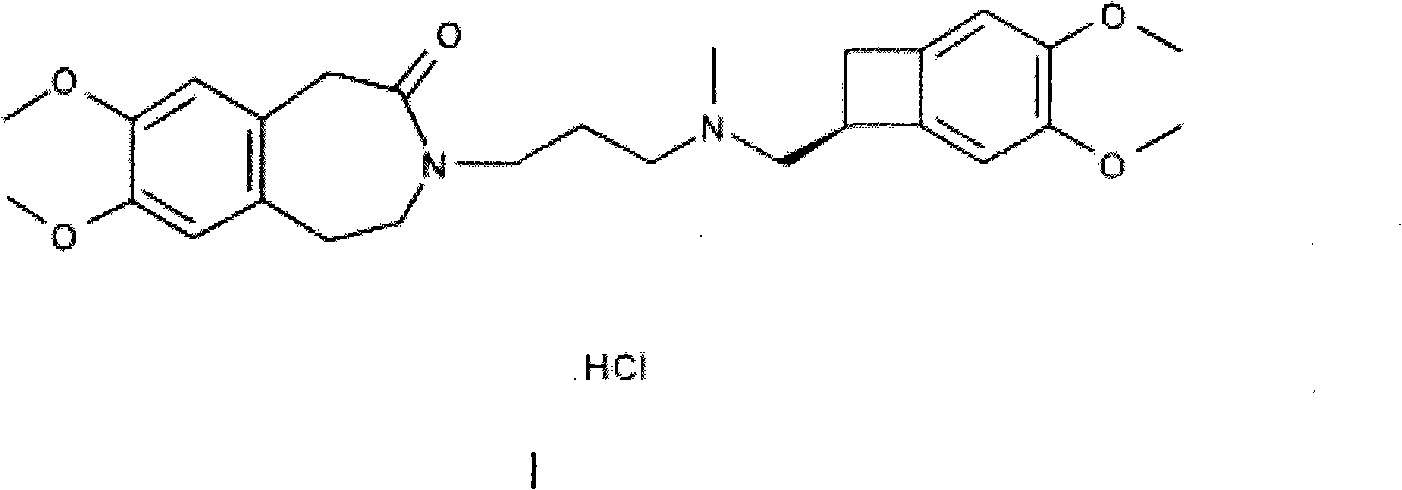
A process for the preparation of Ranolazine (I) and its acid addition salts and the process for the preparation of compound of formula (7).
Owner:UNICHEM LAB LTD
Sustained release pharmaceutical formulations comprising ranolazine
Owner:GILEAD PALO ALTO
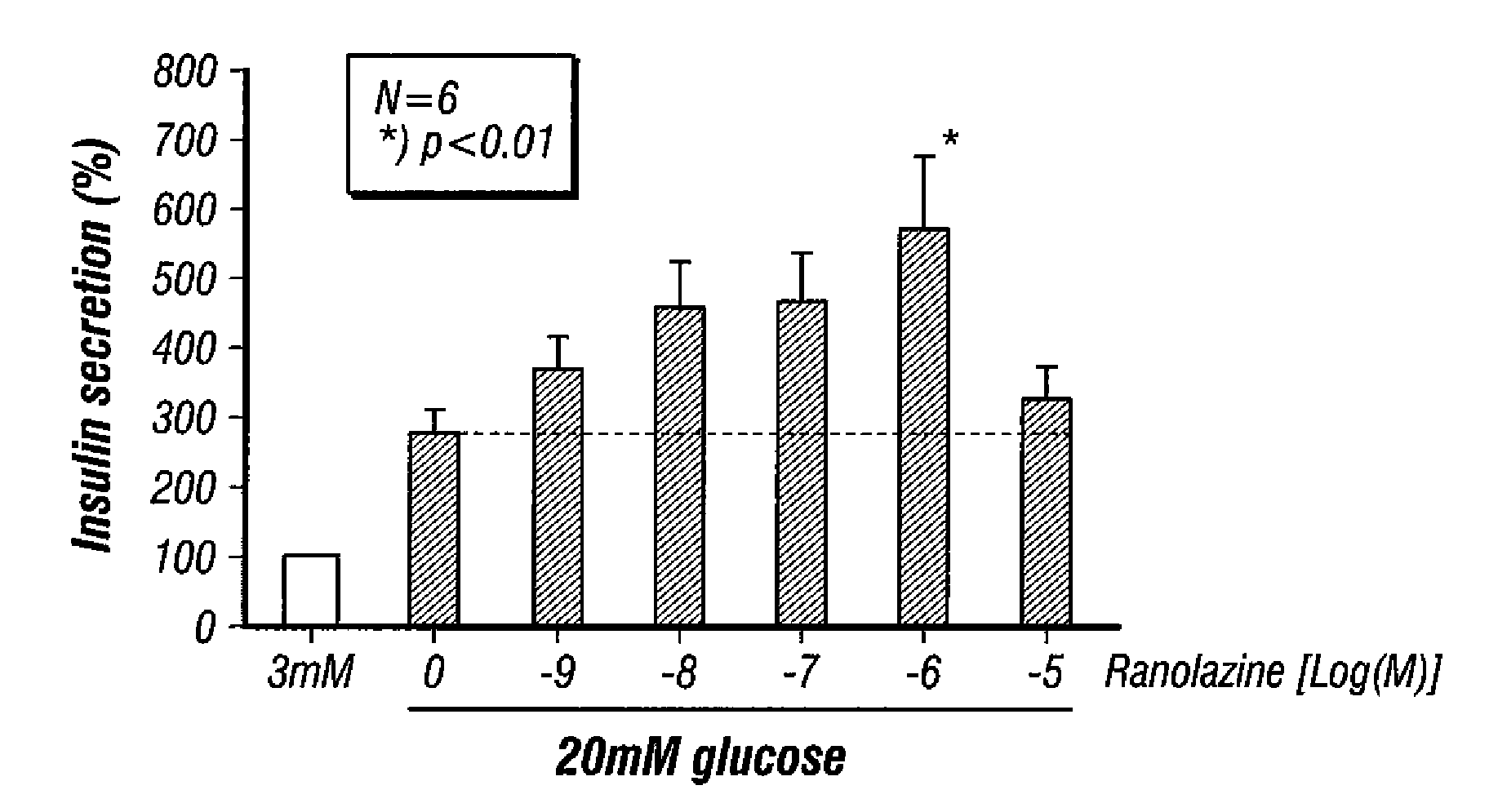
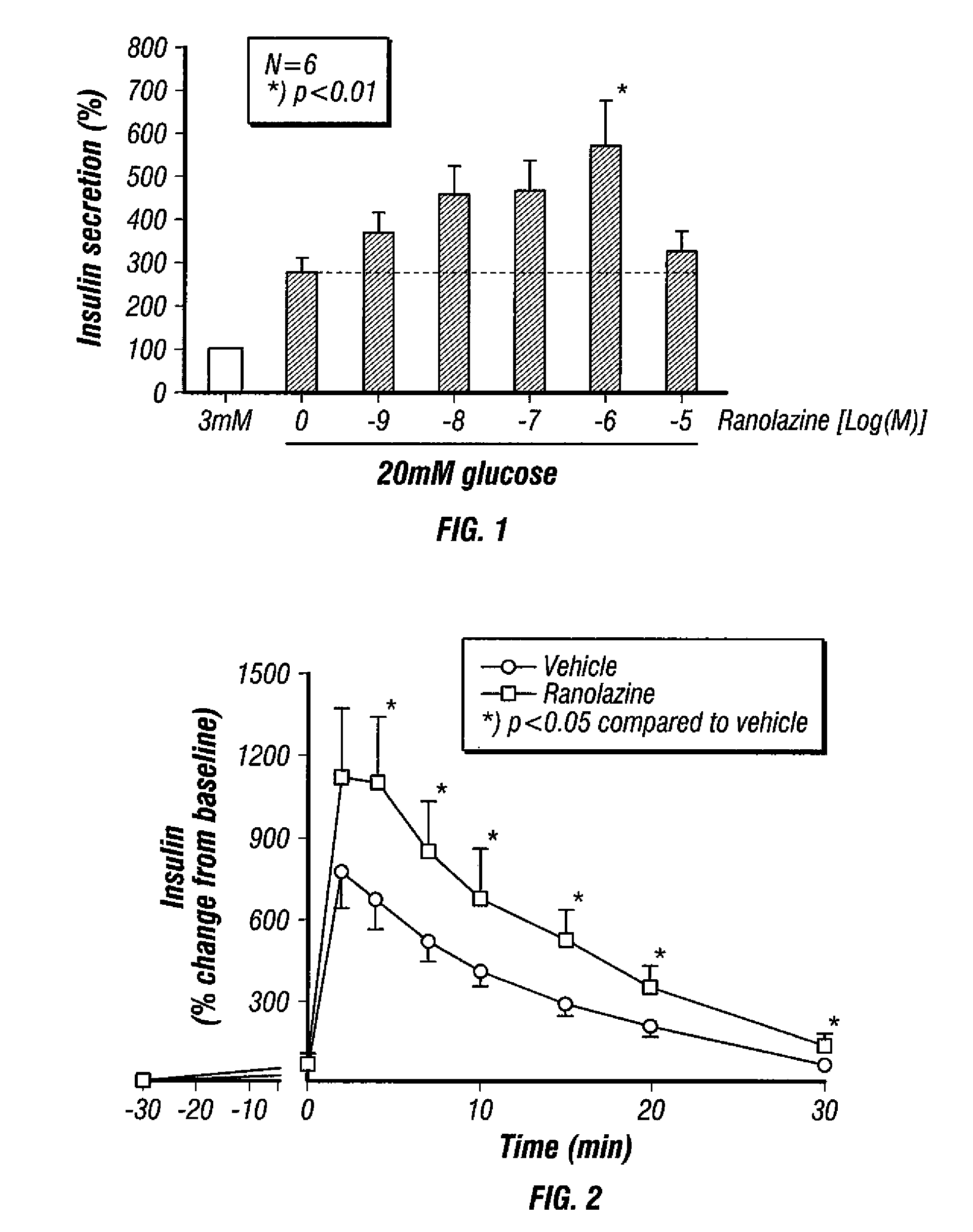
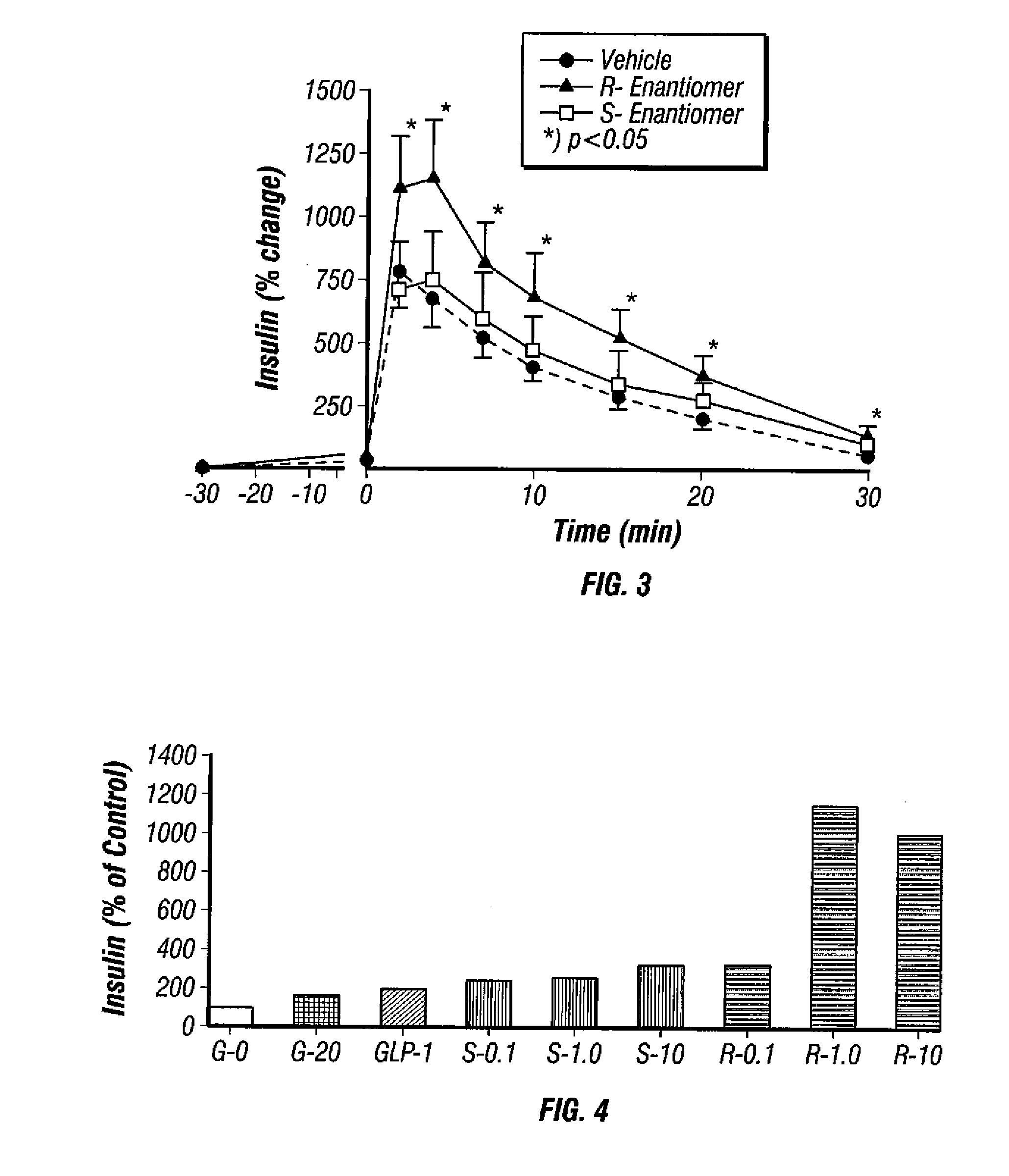
Method for enhancing insulin secretion
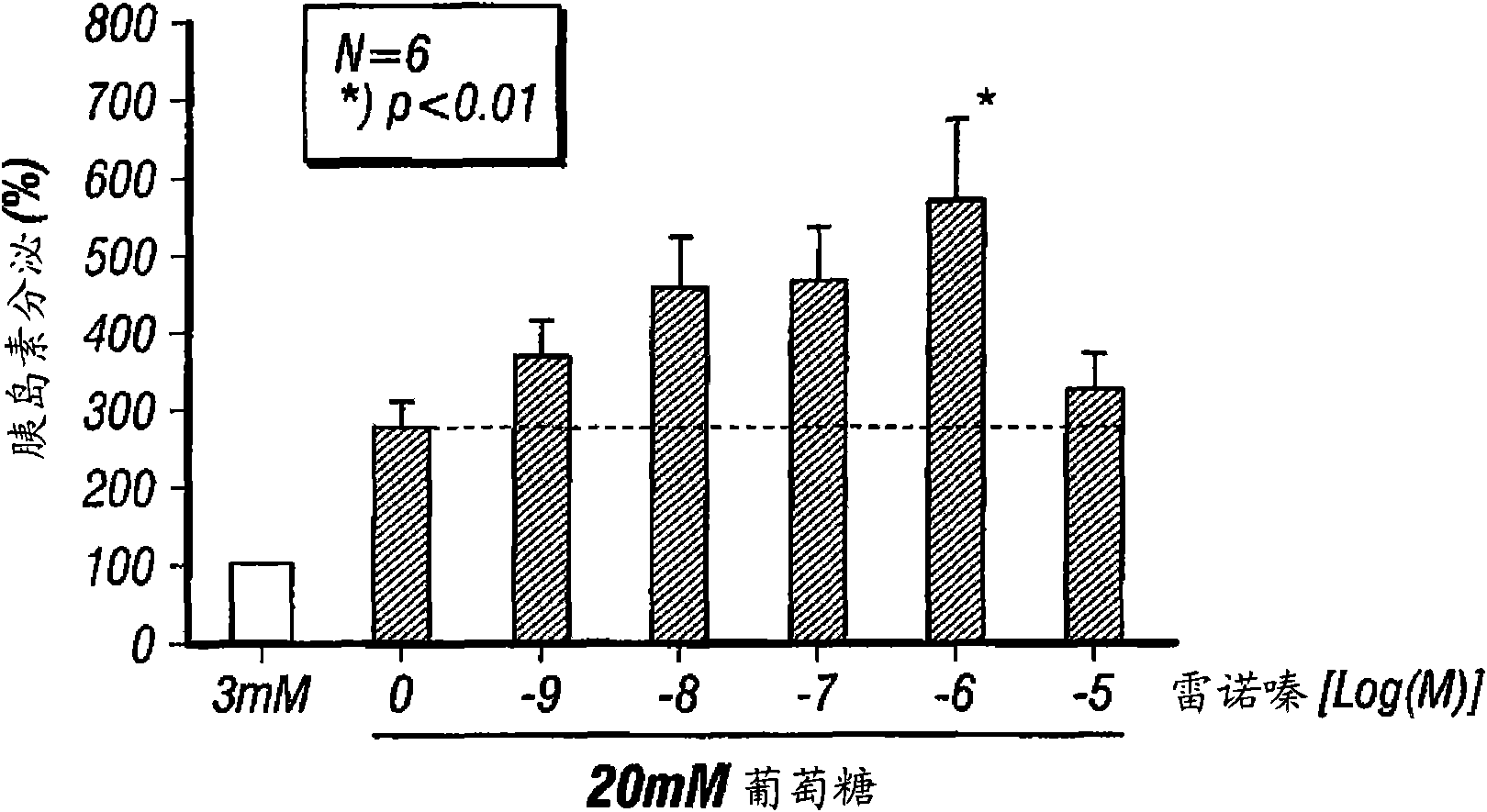
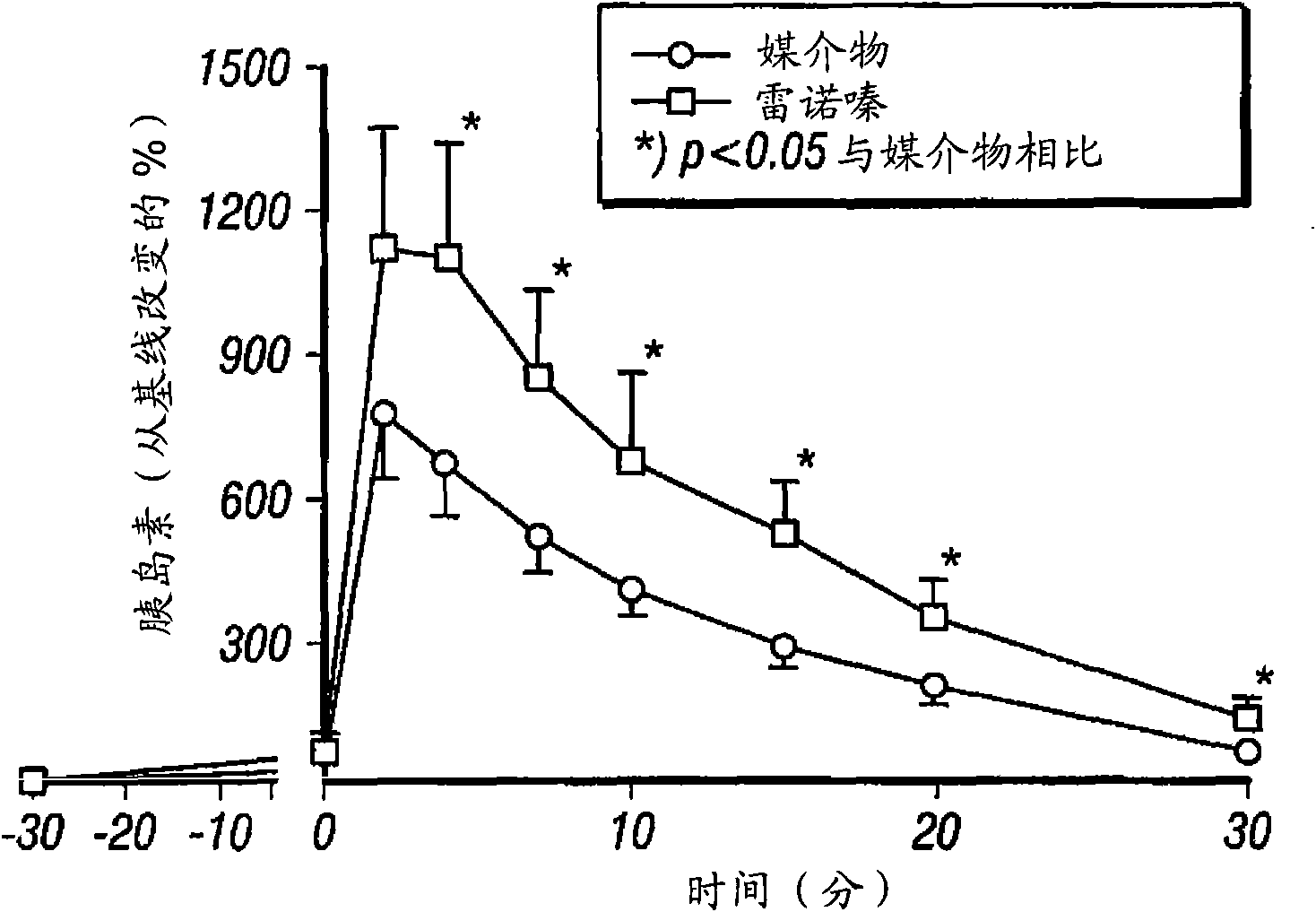
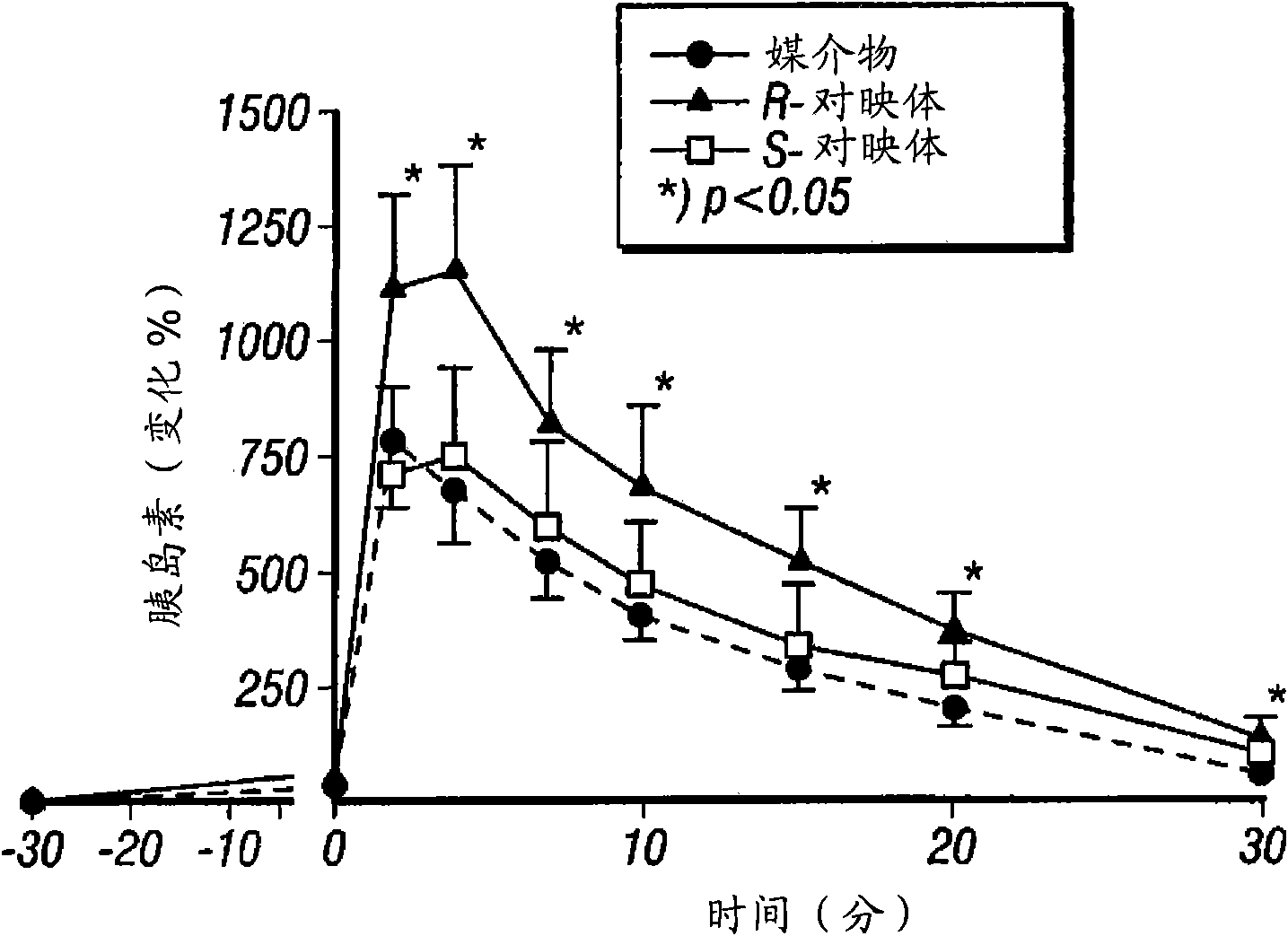
InactiveUS20080255031A1Reducing amount and frequencyReduces adverse eventsOrganic active ingredientsPeptide/protein ingredientsEnantiomerRanolazine
The invention is directed to methods for enhancing endogenous insulin levels in a patient in need thereof which method comprises administering to the patient an insulin secretion-enhancing amount of racemic ranolazine or the R- or S-enantiomer of ranolazine. It is also directed to methods of treatment comprising racemic ranolazine or the R- or S-enantiomer of ranolazine for enhancing endogenous insulin levels in a patient in need thereof. It is also directed to a composition comprising an insulin secretion-enhancing amount of racemic ranolazine or the R- or S-enantiomer of ranolazine and at least one anti-diabetic agent.
Owner:CV THERAPEUTICS INC
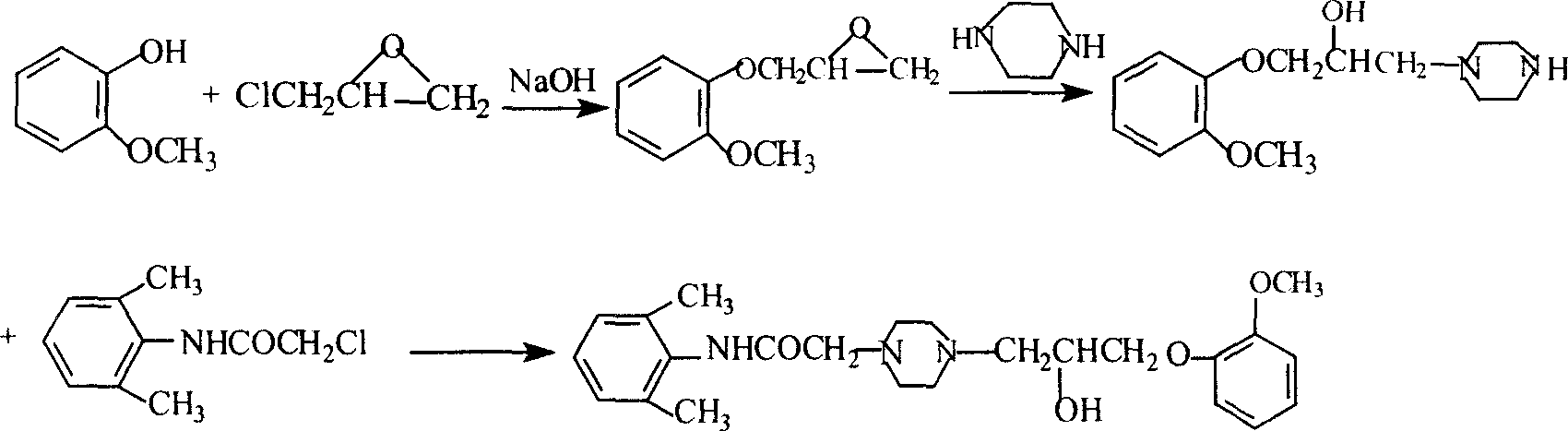
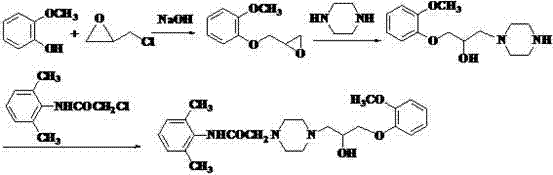
Method for synthesizing ranolazine
InactiveCN101544617AEasy to operateHigh yieldOrganic chemistryCardiovascular disorderRanolazineAminolysis
The invention discloses a method for synthesizing an anti-angina medicament, namely ranolazine, which comprises the following steps that: N-(2,6-dimethylphenyl)chloracetamide is taken as an initial raw material and is condensed with piperazidine, and then the aminolysis reaction of the obtained product and 1-(2-methoxyphenoxyl)-2,3-propylene oxide is performed to obtain a product, namely the ranolazine. During the preparation of an intermediate, namely N-(2,6-dimethylphenyl)-2-(1-piperazinyl)acetamide, the invention improves an operation method, simplifies operation steps, and improves the yield of products.
Owner:FUJIAN TIANQUAN PHARMA
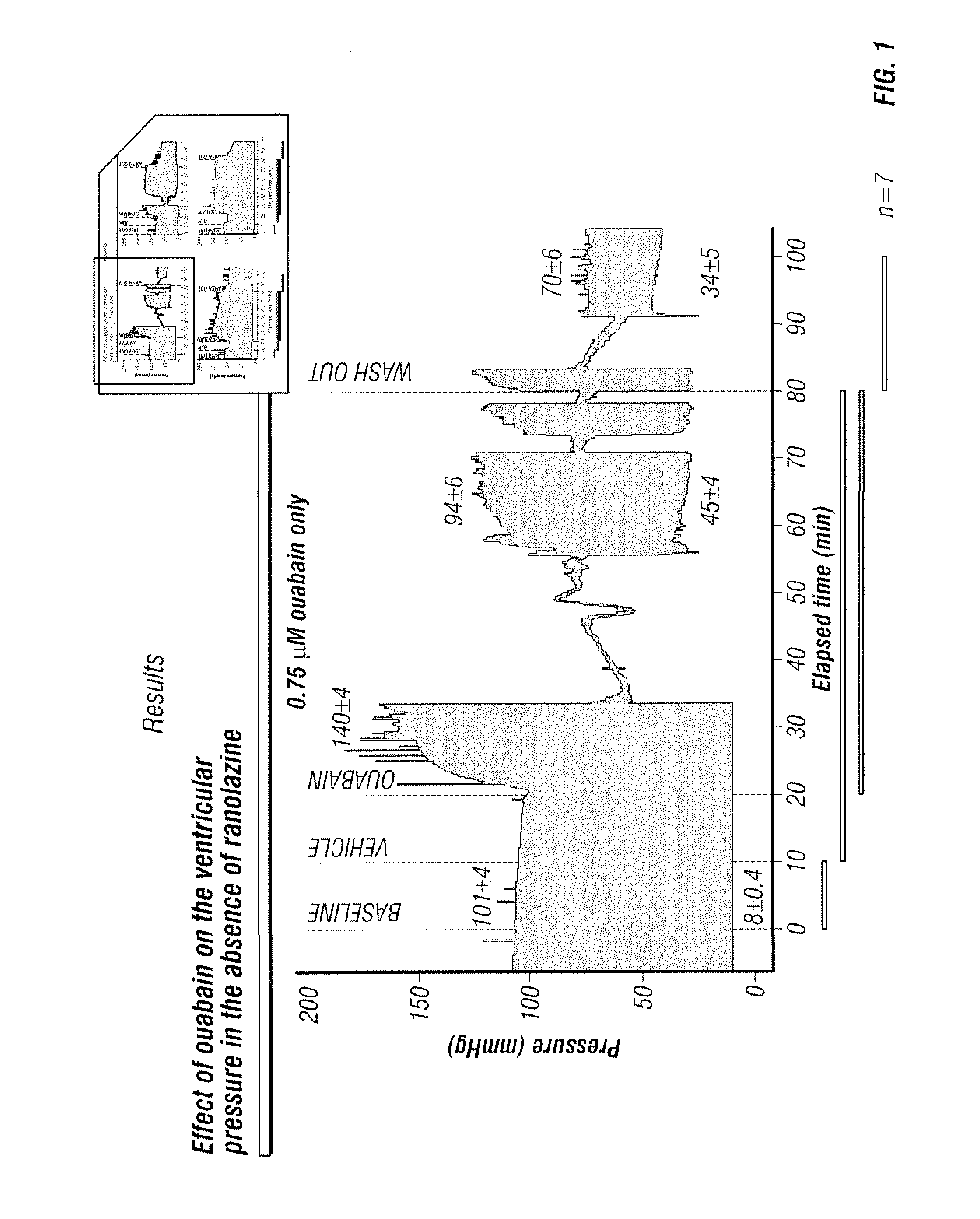
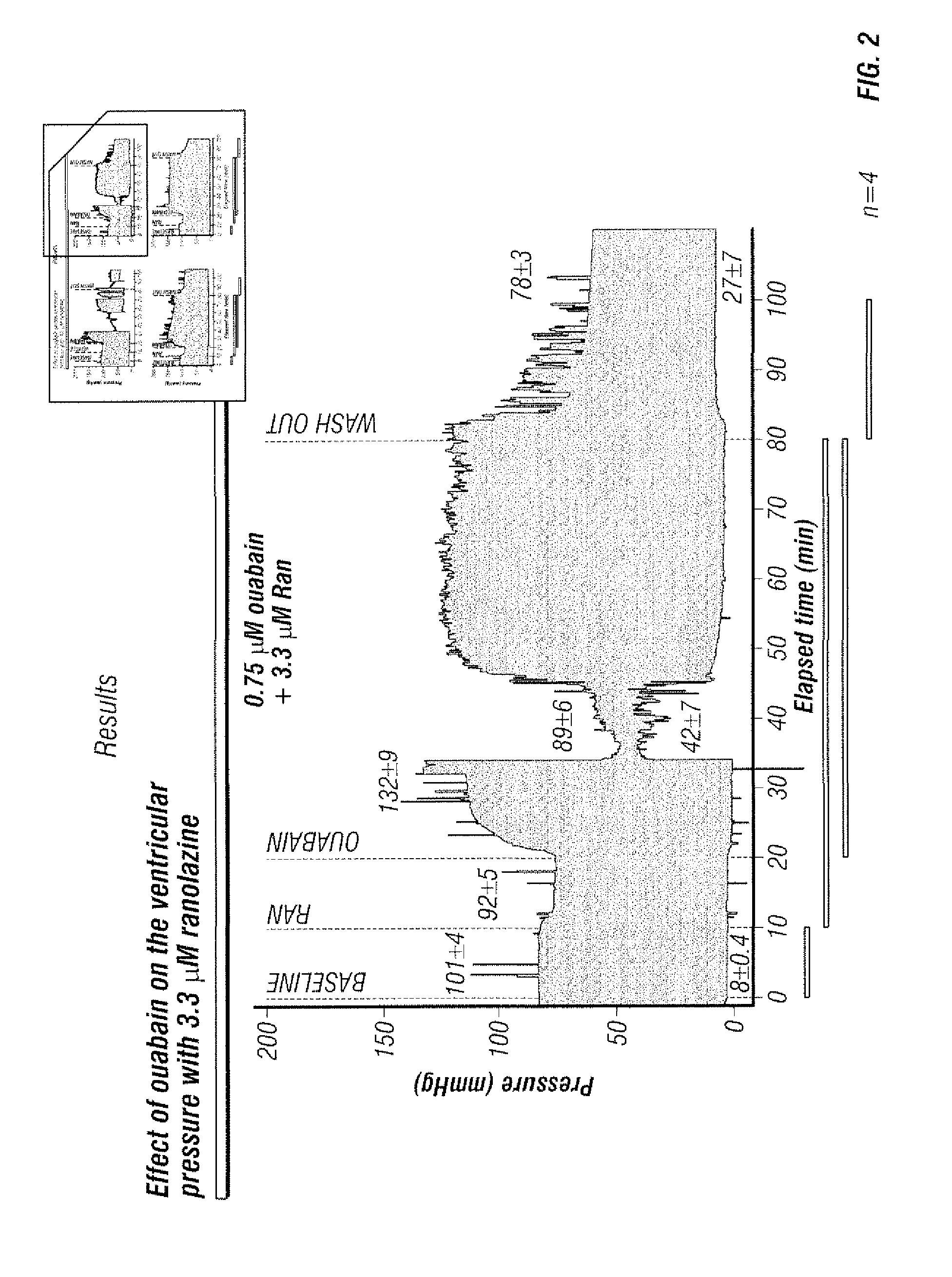
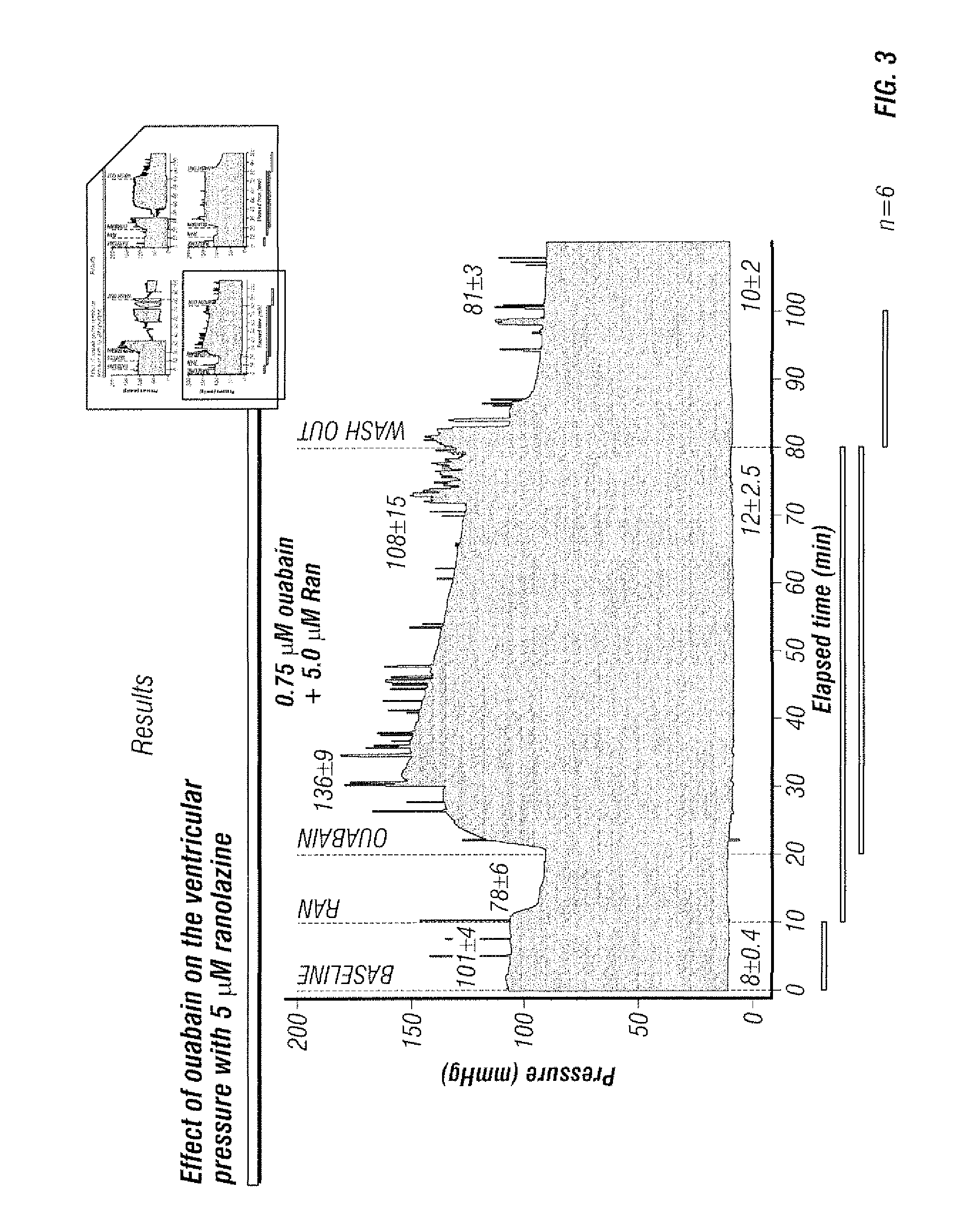
Co-administration of ranolazine and cardiac glycosides
The present invention relates to a method for reducing the toxicity of cardiac glycosides comprising the coadministration of a therapeutically effective amount of cardiac glycoside and a therapeutically effective amount ranolazine. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
Ranolazine sustained release tablet medicine composition and preparation method thereof
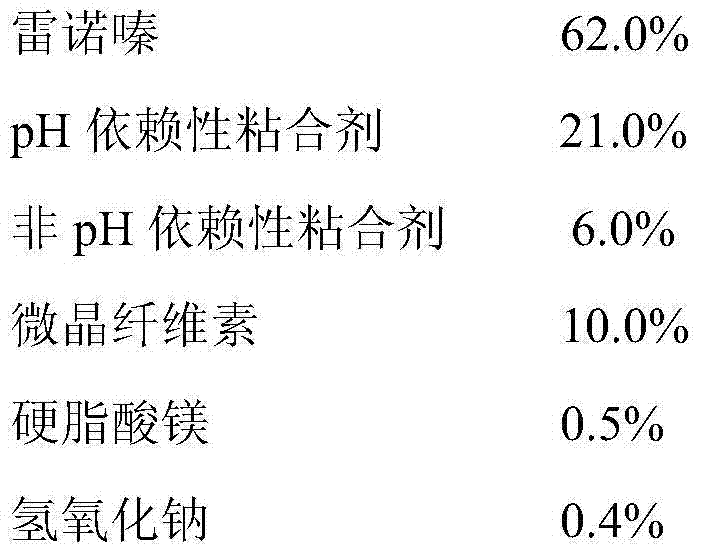
ActiveCN104758265AImprove stabilityImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMagnesium stearate
The invention relates to a ranolazine sustained release tablet medicine composition and a preparation method thereof. The composition comprises, by weight, 50%-72% of ranolazine, 20%-25% of pH dependence binding agents, 5%-8% of non-pH-dependence binding agents, 2%-15% of microcrystalline celluloses, 0.1%-0.5% of magnesium stearate and 0.1%-0.5% of sodium hydroxide. The preparation method includes the steps of mixing, palletizing, particle wrapping, drying, tidying, tabletting, tablet core wrapping and the like. The preparation method s easy and convenient, industrialization is easily achieved, and the prepared ranolazine sustained release tablet medicine composition has the beneficial effects of being good in slow-release effect, light in tablet weight and the like.
Owner:SICHUAN HAISCO PHARMA CO LTD
Ranolazine for enhancing insulin secretion
InactiveCN101657198AReduce adverse eventsOrganic active ingredientsMetabolism disorderLevel insulinRanolazine
The invention is directed to methods for enhancing endogenous insulin levels in a patient in need thereof which method comprises administering to the patient an insulin secretion-enhancing amount of racemic ranolazine or the R- or S-enantiomer of ranolazine. It is also directed to methods of treatment comprising racemic ranolazine or the R- or S-enantiomer of ranolazine for enhancing endogenous insulin levels in a patient in need thereof. It is also directed to a composition comprising an insulin secretion-enhancing amount of racemic ranolazine or the R- or S-enantiomer of ranolazine and at least one anti-diabetic agent.
Owner:GILEAD PALO ALTO
Ranolazine sustained release tablets
The invention relates to a Reynolds pyrazine slow-released tablet, which is characterized in that the tablet comprises not more than 50wt% Reynolds pyrazine and at least an independent adhesive with 1-20wt% low viscosity PH and a or a plurality of independent adhesive with 1-20wt% high viscosity PH.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Slow releasing ranolazine micro pill
The present invention discloses one kind of slow releasing ranolazine micro pill including ranolazine and supplementary material. It consists of coating and isolating layer in 5-30 %, preferably in 10 % and pill core in 70-95 %, preferably in 90 %. The slow releasing ranolazine micro pill is tested to have release amount of 20-40 % within 2 hr, 50-80 % within 6 hr, and 80-100 % within 12 hr.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Oral preparation containing ranolazine hydrochloride for treating cardiovascular disease
InactiveCN1562024AReduce absorptionReduce efficacyOrganic active ingredientsCardiovascular disorderVascular diseaseSoft materials
An orally-applied ranolazine hydrochloride for treating cardiovascular disease is prepared from ranolazine hydrochloride, diluent, adhesive, disintegrant, antisticking agent and lubricant through adding wetting agent, preparing soft material, granulating and drying.
Owner:HUAZHONG NORMAL UNIV
Method for separating ranolazine by adopting simulated moving bed
InactiveCN102952100ASimple processContinuous automated productionSolid sorbent liquid separationOptically-active compound separationCelluloseCarbamate
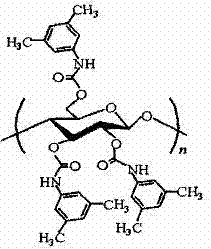
The invention discloses a method for separating chiral compound ranolazine by adopting a simulated moving bed in a fourth region. The method is characterized by adopting a simulated moving bed chromatography system, taking cellulose-tris(3,5-dimethylphenylcarbamate) as a filler and methanol as a mobile phase and separating high-purity R-ranolazine and S-ranolazine from racemes of ranolazine. The simulated moving bed chromatography system has the advantages of continuous production, high degree of automation and high production efficiency.
Owner:JIANGSU HANBON SCI & TECH CO
Medical composition containing ivabradine and ranolazine
ActiveCN101780091AHazard mitigationGood synergyCardiovascular disorderHeterocyclic compound active ingredientsDiseaseCoronary heart disease
The invention provides a medical composition containing ivabradine or pharmacologically acceptable salts and ranolazine or pharmacologically acceptable salts thereof. Proved by research, the medical composition effectively relieves myocardial ischemia and has good synergetic effect on myocardial ischemia diseases, such as angina, coronary heart disease, and the like by the combined use of the ivabradine and the ranolazine. Thus, a method for treating myocardial ischemia diseases with good effect and low adverse reaction is found. The invention finds a good solution for treating myocardial ischemia diseases clinically with poor effect at present.
Owner:LUNAN PHARMA GROUP CORPORATION
Pharmaceutical compositions of ranolazine
Owner:LUPIN LTD
Ranolazine quick-release mini-pill preparation and preparation method thereof
InactiveCN108096219AOrganic active ingredientsPharmaceutical non-active ingredientsOral medicationFluidized bed
The invention provides a ranolazine quick-release mini-pill preparation and a preparation method thereof, and relates to the fields of a medicine preparation technology and application. The administration mode of the quick-release mini-pill preparation is oral administration, a blank pill core serves as a carrier, a ranolazine aqueous solution containing a cosolvent and adhesive is a medicine applying solution, and the use amount of the ranolazine is 0.01 to 0.3 percent of the weight of the pill core according to the weight ratio; and the oral dosage of the ranolazine is not more than 100 micrograms. The ranolazine quick-release mini-pill preparation has the characteristics of high medicine applying rate, high content uniformity, quick medicine release, quick response of analgesic effect and the like. Meanwhile, the quick-release mini-pill preparation has the characteristics of high clinical use compliance, high safety and the like. In addition, the blank pill core fluidized bed medicine applying method is suitable for preparation of a ranolazine medicine oral preparation with extremely low specification.
Owner:FOSHAN TENGRUI MEDICINE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com