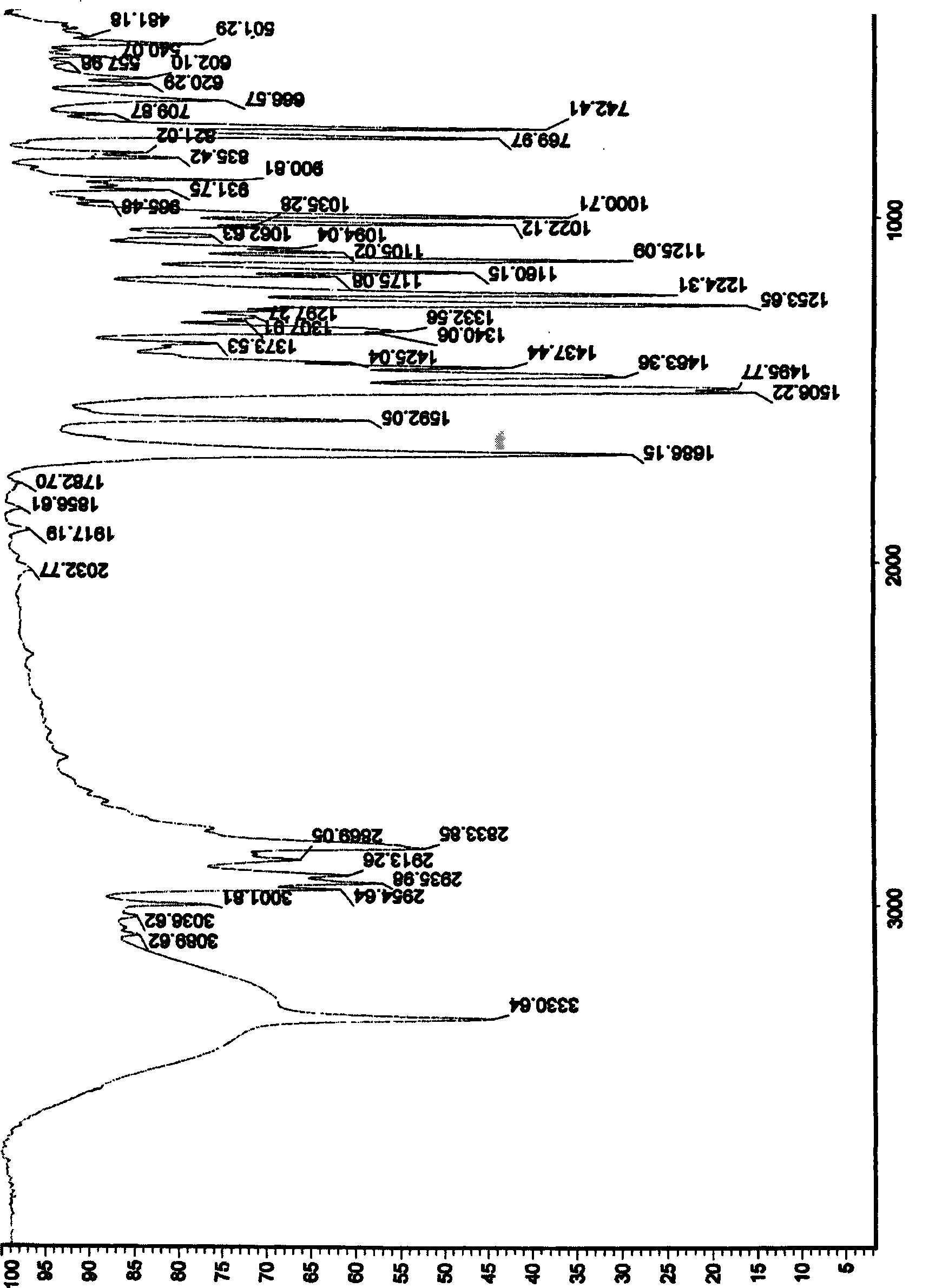
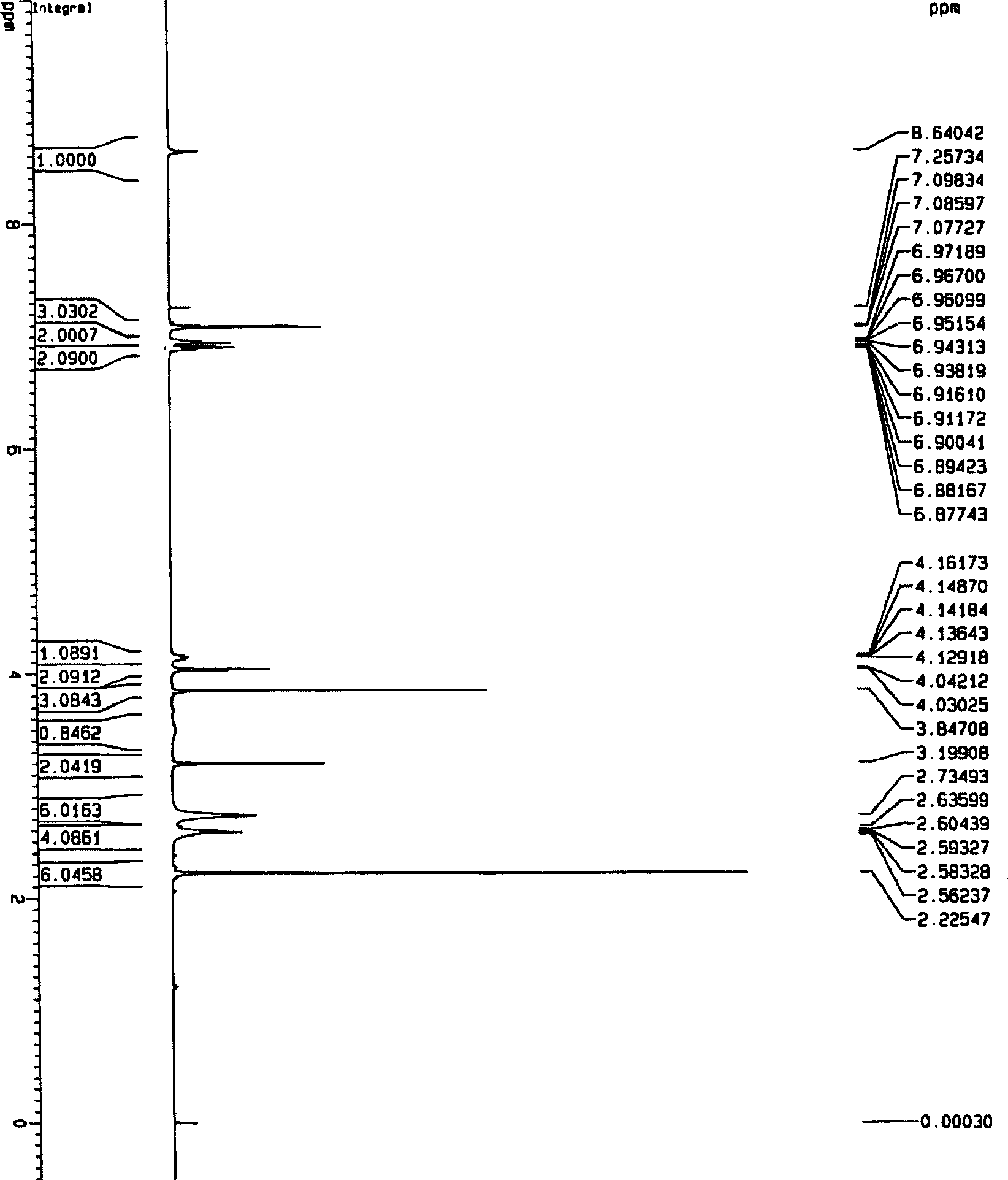
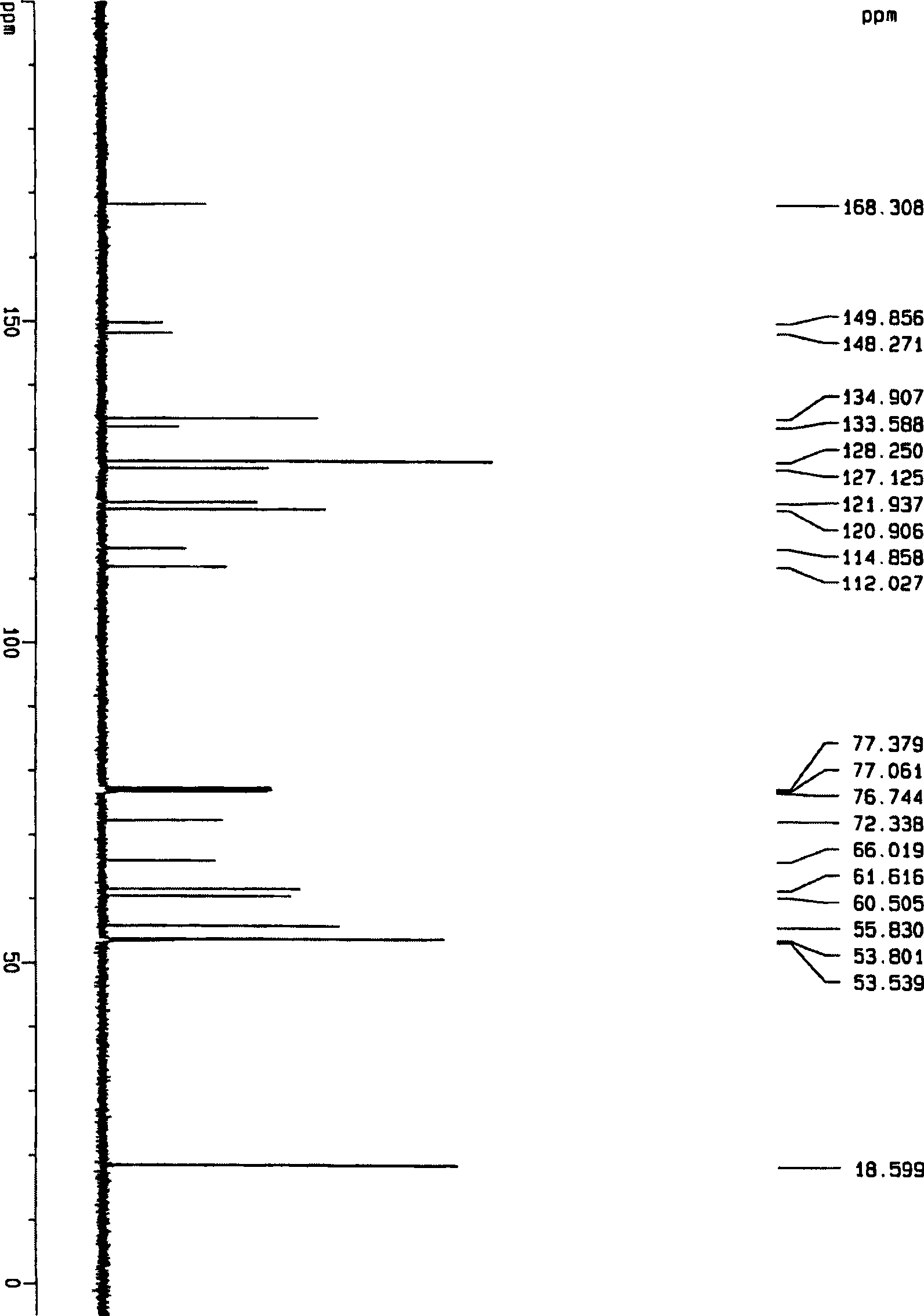
Method for synthesizing ranolazine
A synthesis method and a technology of ranolazine are applied in the synthesis field of anti-angina pectoris drug ranolazine, can solve the problems of difficulty in industrialized production, low yield, complicated operation and the like, and achieve the effects of good quality, high yield and simplified operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Preparation of N-(2,6-dimethylphenyl)-2-(1-piperazinyl)acetamide
[0029] Add 60g of N-(2,6-dimethylphenyl) chloroacetamide, 103.2g of anhydrous piperazine and 600ml of absolute ethanol in the reaction flask, and stir the reaction mixture under reflux for 3hr. After the reaction is completed, cool to room temperature and add ammonia water Adjust the pH value to 9, add 500ml of ethyl acetate after concentrating under reduced pressure to recover the solvent, stir to dissolve, filter, concentrate and recover the solvent under reduced pressure to dryness, then add 750ml of anhydrous ether, stir to complete the precipitation, filter, take the precipitate and dry it to obtain white solid product. mp: 109-111°C.
[0030] 2) preparation of ranolazine crude product
[0031] Add 60g N-(2,6-dimethylphenyl)-1-piperazine acetamide and 48g 1-(2-methoxyphenoxy group)-2,3-propylene oxide in the reaction flask, and then After adding 600 ml of absolute ethanol, the reaction mixture...
Embodiment 2
[0036] 1) Preparation of N-(2,6-dimethylphenyl)-2-(1-piperazinyl)acetamide
[0037] Add 66g of N-(2,6-dimethylphenyl) chloroacetamide, 90g of anhydrous piperazine and 600ml of absolute ethanol in the reaction flask, stir the reaction mixture and reflux for 5hr, after the reaction is completed, cool to room temperature, add ammonia water to adjust The pH value is 10, add 500ml of ethyl acetate after concentrating under reduced pressure to recover the solvent, stir to dissolve, filter, concentrate under reduced pressure and recover the solvent to dryness, then add 750ml of anhydrous ether, stir to complete the precipitation, filter, take the precipitate and dry to obtain a white solid product. mp: 109-111°C.
[0038] 2) preparation of ranolazine crude product
[0039] Add 72g N-(2,6-dimethylphenyl)-1-piperazine acetamide and 36g 1-(2-methoxyphenoxy group)-2,3-propylene oxide in the reaction flask, and then 600ml of absolute ethanol was added, and the reaction mixture was stir...
Embodiment 3
[0044] 1) Preparation of N-(2,6-dimethylphenyl)-2-(1-piperazinyl)acetamide
[0045] Add 54g of N-(2,6-dimethylphenyl) chloroacetamide, 108g of anhydrous piperazine and 600ml of absolute ethanol in the reaction flask, stir the reaction mixture and reflux for 4hr, after the reaction is completed, cool to room temperature, add ammonia water to adjust The pH value is 9, add 500ml of ethyl acetate after concentrating under reduced pressure to recover the solvent, stir to dissolve, filter, concentrate under reduced pressure and recover the solvent to dryness, then add 750ml of anhydrous ether, stir to complete the precipitation, filter, take the precipitate and dry to obtain a white solid product. mp: 109-111°C.
[0046] 2) preparation of ranolazine crude product
[0047]Add 48g N-(2,6-dimethylphenyl)-1-piperazine acetamide and 60g 1-(2-methoxyphenoxy group)-2,3-propylene oxide in the reaction flask, and then 600ml of absolute ethanol was added, and the reaction mixture was stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com