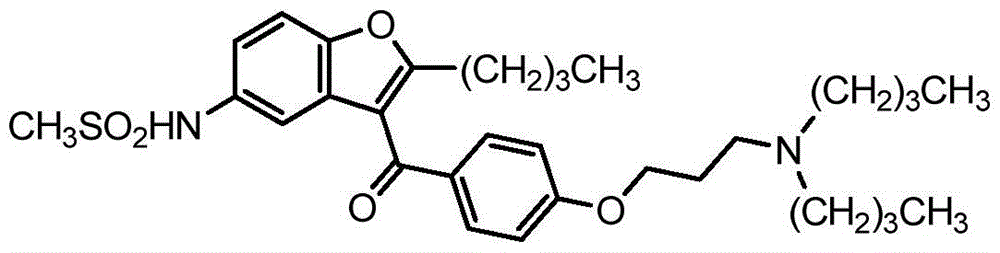
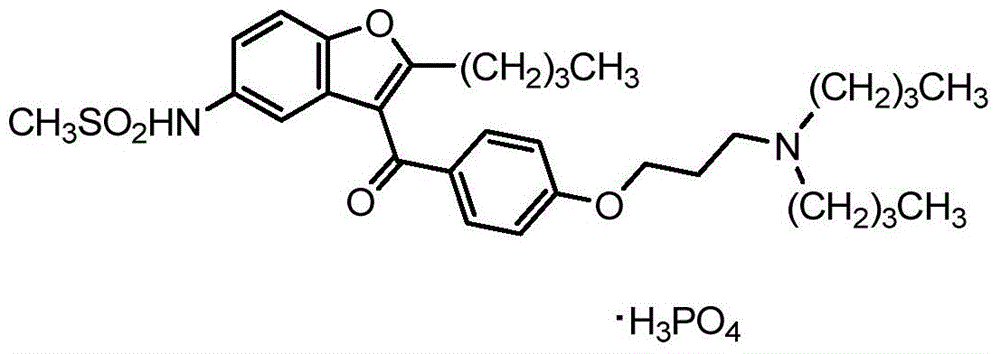
Pharmaceutical compositions of ranolazine and dronedarone
一种决奈达隆、决奈达隆碱的技术,应用在雷诺嗪和决奈达隆的药物组合物领域,能够解决心悸或胸痛等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Preparation operation
[0133] For the preparation of spray-dried solid dispersions (formulations) of dronedarone in the form of phosphate salt formulations, the equipment train consisted of a glass reactor, a spray dryer (MobileMinor ,GEANiro, Denmark) and a tray drying vacuum oven.
[0134] Preparation of Feed Solutions
[0135] A batch of dronedarone feed solution at 15.0% (w / w) solids content was prepared on a scale of 62.8 kg solution, corresponding to 9.42 kg spray-dried powder. Drug solution and polymer solution were prepared separately in two glass reactors. To prepare the drug solution, the dronedarone drug substance is dispersed in a dilute phosphoric acid solution and gradually dissolves due to its reaction with phosphoric acid. Initially, 95% of theoretical phosphoric acid was added to prepare the feed solution. After the drug solution was dissolved, the pH of the drug solution was adjusted to 4.0 ± 0.4 with the remaining phosphoric acid solution. Not...
Embodiment 2
[0147] method
[0148] Feed solutions for laboratory experiments were prepared by: 1) preparing an aqueous solution of counterions (e.g., phosphate, citrate, acetate); 2) adding dronedar to the acid solution from the previous step 3) Separately prepare an aqueous solution of the polymer (eg, HPMCE3LV, HPMCE5LV, PVP, PVPVA, or HPMCAS); and (4) Combine the solutions from steps (2) and (3). The total solids content of the feed solution ranges from about 10% to 20% w / w. Optionally, the feed solution can be prepared by stepwise addition of the ingredients (phosphoric acid, dronedarone and polymer) to the chosen solvent.
[0149] Spray drying feed solution:
[0150] The dronedarone feed solution was spray dried using a Buchi Mini Spray Dryer B-290 in a sealed ring configuration. Compressed nitrogen was used as both drying and nebulizing gas. Dry gas fans run at 100% power. The temperature of the condenser was set at about 4°C to remove water from the recirculated drying gas. T...
Embodiment 2A
[0164] method
[0165] Additional laboratory experiments were performed to develop various feed solution compositions. Feed solutions for laboratory experiments were prepared by (1) preparing solutions of phosphoric acid in water and / or solvents; (2) dry blending dronedarone and polymer (HPMCE3); (3) ) adding the dry blend to the phosphoric acid solution from step (1); the total solids content of the feed solution ranges from about 15% to 30% w / w. Optionally, the feed solution can be prepared by stepwise addition of ingredients (phosphoric acid, dronedarone and polymer) to the chosen solvent.
[0166] Spray drying feed solution:
[0167] Feed solution of dronedarone with Mini Spray Dryer B-290 (Buchi Corporation) spray dried in a sealed or open ring configuration. Compressed nitrogen was used as both drying and nebulizing gas. Dry gas fans run at 100% power. The temperature of the condenser was set at about 4°C to 10°C to remove water from the recirculated drying gas. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com