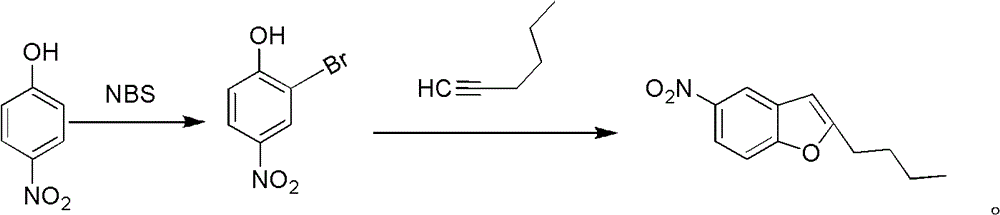
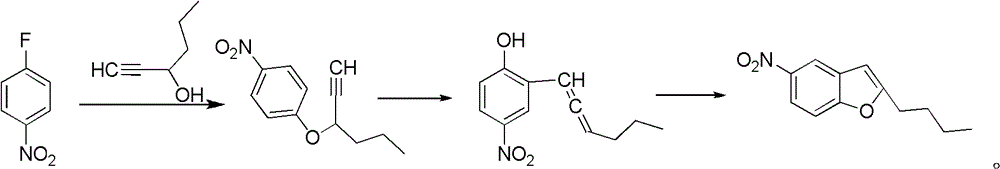
Method for synthesis of dronedarone
A synthesis method and technology of dronedarone hydrochloride are applied in the field of synthetic process routes of the drug compound dronedarone, can solve the problems of trivial yield, low yield, low yield and the like, and achieve trivial operation and high yield. The effect of high rate and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
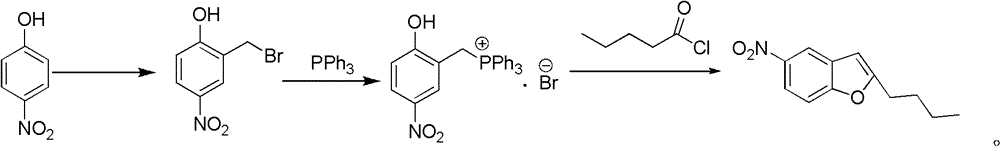
[0044] 1, the preparation of 2-chloromethyl-4-nitrophenol
[0045] Add 60g of p-nitrophenol, 26.16g of paraformaldehyde, 432mL of concentrated hydrochloric acid and 5mL of concentrated HCl into a 1L three-neck flask 2 SO 4 , stirred, heated to 88 ~ 90 ° C, and reacted at this temperature for 4h. The reaction solution was cooled to room temperature, filtered, and the resulting solid was dried under reduced pressure to obtain 75.47 g of off-white to off-white solid, mp 121-125°C.
[0046] 2. Preparation of 2-hydroxy-5-nitrobenzyltriphenylphosphonium chloride
[0047] Add 50g of 2-chloromethyl-4-nitrophenol, 69.80g of triphenylphosphine and 1500mL of chloroform into a 3L three-necked flask, and heat to reflux for 1h. The reaction liquid was cooled to normal temperature, filtered, and dried to obtain 115.63 g of off-white to white solid, yield 96.4%, mp 262-265°C.
[0048] 3. Preparation of 2-n-butyl 5-nitrobenzofuran
[0049]Add 400mL of toluene, 45.4g of triethylamine, 60.1...
Embodiment 2
[0063] 1, the preparation of 2-chloromethyl-4-nitrophenol
[0064] Add 45g of p-nitrophenol, 19.6g of paraformaldehyde, 324mL of concentrated hydrochloric acid and 3mL of phosphoric acid into a 1L three-necked flask, stir, heat to 88-90°C, and react at this temperature for 4h. The reaction solution was cooled to room temperature, filtered, and the resulting solid was dried under reduced pressure to obtain 36.5 g of off-white to off-white solid, mp 124-127°C.
[0065] 2. Preparation of 2-hydroxy-5-nitrobenzyltriphenylphosphonium chloride
[0066] Add 50g of 2-chloromethyl-4-nitrophenol, 69.80g of triphenylphosphine and 1500mL of chloroform into a 3L three-necked flask, and heat to reflux for 1h. The reaction liquid was cooled to normal temperature, filtered, and dried to obtain 115.63 g of off-white to white solid, yield 96.4%, mp 262-265°C.
[0067] 3. Preparation of 2-n-butyl 5-nitrobenzofuran
[0068] Add 400mL of toluene, 45.4g of triethylamine, 60.11g of 2-hydroxy-5-nit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com