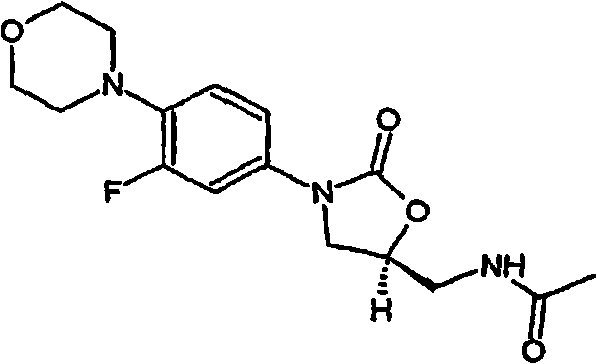
Preparation method of linezolid and preparation thereof
A technology for linezolid and preparations is applied in the field of preparation of linezolid and lyophilized powder injection preparations, which can solve the problems of inconvenient transportation of injections, easy freezing of injections, influence on stability, etc. Long shelf life and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Step 1: Synthesis of 2(S)-1-amino-3-chloro-2-propanol hydrochloride: In a 100ml three-necked flask, add 10.07g benzaldehyde, 30ml ethanol, 10.26g 25% ammonia water, and then drop slowly Add 10.19g (S)-epichlorohydrin, stir at room temperature for 15h, concentrate, add 37% HCl 10.84g and 10ml water, stir at 35°C to 45°C for 2h, separate the water layer, concentrate, ethanol-petroleum ether crystallization, Suction filtration, washing, and drying yielded 3.67 g of white solid;
[0051] Step 2: Synthesis of (S)-N-[2-acetoxy-3-chloropropyl]acetamide: In a 50ml three-necked flask, add 2.08g of compound 2(S)-1-amino-3-chloro- 2-propanol hydrochloride, 10ml of CH 2 Cl 2 , 5.37 g of Ac 2 O, 2.61g of pyridine was dropped into it at 30°C. After the dropwise addition was completed, the reaction was kept for 15h; at 6°C, 5.27g of K was added slowly. 2 CO 3 20ml of aqueous solution, the organic layer was separated, and the aqueous layer was washed with CH 2 Cl 2 Extract, comb...
Embodiment 2
[0058] Step 1: Synthesis of 2(S)-1-amino-3-chloro-2-propanol hydrochloride
[0059] In a 100ml three-necked flask, add 19.98g benzaldehyde, 40ml ethanol, 19.94g 25% ammonia water, then slowly drop in 18.76g (S)-epichlorohydrin, stir at room temperature for 255h, concentrate, add 37% HCl19.34g and 20ml of water, stirred at 35°C-45°C for 4h, separated the water layer, concentrated, crystallized with ethanol-petroleum ether, filtered with suction, washed, dried to obtain 4.57g of white solid;
[0060] Step 2: Synthesis of (S)-N-[2-acetoxy-3-chloropropyl]acetamide
[0061] In a 50ml three-necked flask, add 4.03g compound 2(S)-1-amino-3-chloro-2-propanol hydrochloride, 20ml CH 2 Cl 2 , 14.49 g Ac 2 O, 6.73g of pyridine was dropped into it at 30°C. After the dropwise addition was completed, the reaction was kept for 25h; at 6°C, slowly added 9.96g of K 2 CO 3 40ml of aqueous solution, the organic layer was separated, and the aqueous layer was washed with CH 2 Cl 2 Extract, co...
Embodiment 3
[0070] Step 1: Synthesis of 2(S)-1-amino-3-chloro-2-propanol hydrochloride
[0071] In a 100ml three-necked flask, add 13.90g benzaldehyde, 35ml ethanol, 13.60g 25% ammonia water, then slowly drop 11.80g (S)-epichlorohydrin, stir at room temperature for 20h, concentrate, add 37% HCl19.10g and 18ml of water, stirred at 35°C to 45°C for 3h, separated the water layer, concentrated, crystallized with ethanol-petroleum ether, filtered with suction, washed, and dried to obtain 14.10g of white solid;
[0072] Step 2: Synthesis of (S)-N-[2-acetoxy-3-chloropropyl]acetamide
[0073] In a 50ml three-necked flask, add 5.00g compound 2(S)-1-amino-3-chloro-2-propanol hydrochloride, 18mlCH 2 Cl 2 , 8.00gAc 2 O, 3.40g of pyridine was dropped into it at 30°C, and the dropwise addition was completed, and the reaction was kept for 20h. At 6°C, slowly add 9.30gK 2 CO 3 30ml of aqueous solution, the organic layer was separated, and the aqueous layer was washed with CH 2 Cl 2 Extract, combi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com