Transition metal complex based on semirigid bipyridine bisamide organic ligand and terephthalic acid as well as synthetic method and application of transition metal complex
A technology of bispyridine bisamide and terephthalic acid, applied in the fields of cadmium organic compounds, zinc organic compounds, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
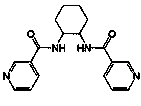
[0052] Example 1 Synthesis of [Zn(3-bpah)(1,4-BDC)]·H 2 O, where 3-bpah is N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, the structural formula is: , 1,4-BDC is terephthalate
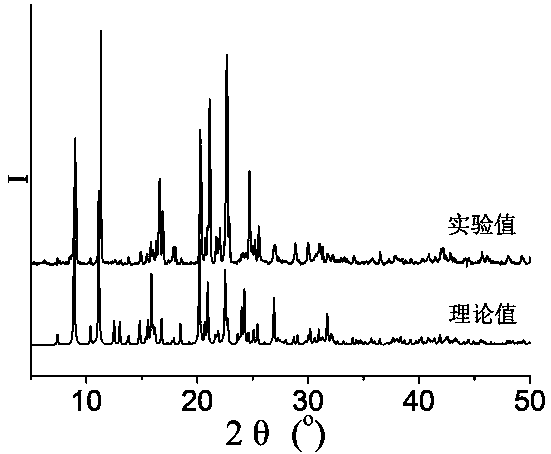
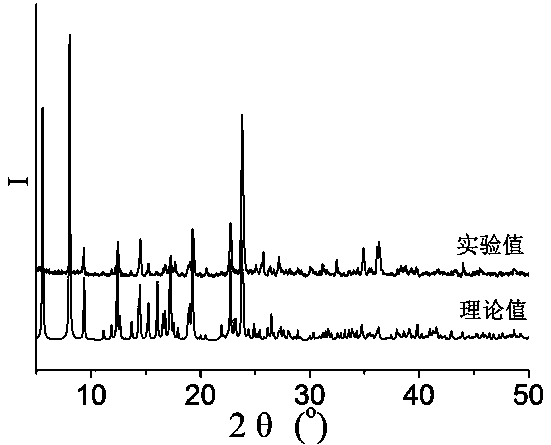
[0053] 0.3mmol Zn(NO 3 ) 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 0.10 mmol terephthalic acid and 8.0 mL H 2 O was added to a 25mL beaker in turn, stirred at room temperature for 40min, adjusted to pH 6.5 with 0.1mol / L NaOH solution, poured into a 25mL autoclave, heated to 100°C at a heating rate of 5°C / h, and heated with water Under the condition of heat preservation for 48h, the temperature was lowered to room temperature at a cooling rate of 5°C / h to obtain colorless blocky crystals, which were dried naturally at room temperature to obtain [Zn(3-bpah)(1,4-BDC)]·H 2 O, the yield is 48%, and its coordination environment diagram is as follows Figure 4 As shown, its two-dimensional network diagram is shown as Figure 5 As shown, the simplified diagram of i...
Embodiment 2
[0054] Example 2 Synthesis of [Zn(3-bpah)(1,4-BDC)]·H 2 O, where 3-bpah is N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 1,4-BDC as terephthalate
[0055] 0.2 mmol Zn(NO 3 ) 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 0.15 mmol terephthalic acid and 10.0 mL H 2 Add O to a 25mL beaker in turn, stir at room temperature for 30min, adjust the pH to 6.8 with 1mol / L NaOH solution, pour it into a 25mL autoclave, and raise the temperature to 110°C at a heating rate of 10°C / h. Keep it warm for 36 hours, and lower the temperature to room temperature at a cooling rate of 10 °C / h to obtain colorless blocky crystals, which are naturally dried at room temperature to obtain [Zn(3-bpah)(1,4-BDC)] H 2 O, the yield is 56%, and its coordination environment diagram is as follows Figure 4 As shown, its two-dimensional network diagram is shown as Figure 5 As shown, the simplified diagram of its two-dimensional skeleton structure is shown in Image 6 sho...
Embodiment 3
[0056] Example 3 Synthesis of [Zn(3-bpah)(1,4-BDC)]·H 2 O, where 3-bpah is N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 1,4-BDC as terephthalate
[0057] 0.2 mmol Zn(NO 3 ) 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 0.15mmol terephthalic acid and 13.0mL H 2 Add O to a 25mL beaker in turn, stir at room temperature for 20min, adjust the pH to 7.2 with 2mol / L NaOH solution, pour it into a 25mL autoclave, and raise the temperature to 120°C at a heating rate of 5°C / h. Keep it warm for 24 hours, and lower the temperature to room temperature at a cooling rate of 10 °C / h to obtain colorless blocky crystals, which are naturally dried at room temperature to obtain [Zn(3-bpah)(1,4-BDC)] H 2 O, the yield is 66%, and its coordination environment diagram is as follows Figure 4 As shown, its two-dimensional network diagram is shown as Figure 5 As shown, the simplified diagram of its two-dimensional skeleton structure is shown in Image 6 shown....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com