Intermediate and method used for preparing dronedarone
A technology for dronedarone and intermediates, which is applied in the field of intermediates for the preparation of dronedarone, and can solve the problems of strict requirements on reaction conditions, increased costs, and unsuitability for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
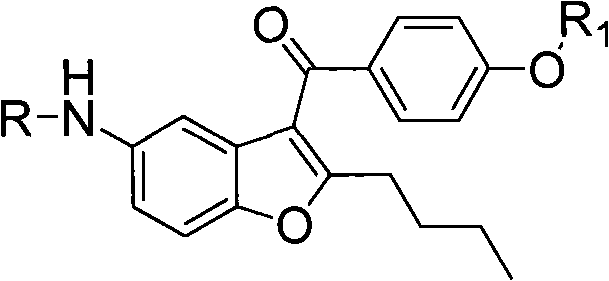
[0060] Intermediates for the preparation of dronedarone and its synthesis process:
[0061]
[0062] Wherein R is acetyl or methanesulfonyl, R1 is H, methyl or ethyl.
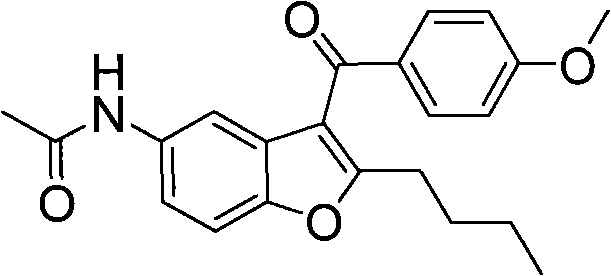
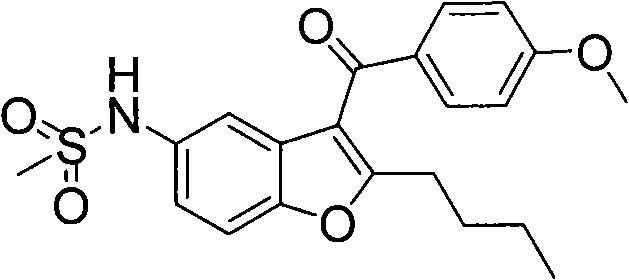
[0063] The chemical names of the intermediates of dronedarone in this example are 2-n-butyl-3-(4-methoxy-benzoyl)-5-acetamidobenzofuran (compound 2), 2- n-Butyl-3-(4-methoxy-benzoyl)-5-methanesulfonylaminobenzofuran (compound 4), 2-n-butyl-3-(4-hydroxy-benzoyl)- 5-Acetamidobenzofuran (Compound 8) 9). The structural formula is as follows:
[0064]
[0065] A synthetic method for preparing an intermediate of dronedarone medicine, which comprises the following steps:
[0066] 1) Compound (1) reacts with p-methoxybenzoyl chloride under the catalysis of up to 5 equivalents of Lewis acid to obtain compound (2);
[0067] 2) compound (2) is obtained under the action of 10% to 36% concentrated hydrochloric acid to obtain compound (3);
[0068] 3) Compound (3) is obtained under the action of methanesulfonyl c...
Embodiment 2
[0092] Preparation of 2-n-butyl-3-(4-methoxy-benzoyl)-5-acetamidobenzofuran (Compound 2)
[0093]
[0094] Weigh 5g (21.6mmol) of 2-n-butyl-5-acetamidobenzofuran (compound 1) and 3.1g (23.8mmol) of p-methoxybenzoyl chloride into a 250ml three-necked flask, and use 40ml The dichloromethane was dissolved, cooled to 0-5 degrees with an ice-water bath, and 14.2g (54..7mmol) of tin tetrachloride was slowly added dropwise at this temperature. After the dropwise addition, stir at this temperature for 1 h and then at room temperature for 2 h. Then 100ml of water was added dropwise to the system, separated, the aqueous phase was extracted with 50ml of dichloromethane, the organic phase was combined, the organic phase was washed with saturated aqueous sodium carbonate (50ml*2), and dried over anhydrous sodium sulfate. Ethyl acetate: n-hexane 1:1 silica gel column separation to obtain 5.1 g of white solid compound (2) with a yield of 65%.
[0095] HNMR (CDCl 3 )δ: 0.85(t, 3H), 1.31...
Embodiment 3
[0097] Preparation of 2-n-butyl-3-(4-methoxy-benzoyl)-5-methanesulfonylaminobenzofuran (Compound 4)
[0098]
[0099] Weigh 5g (13..7mmol) 2-n-butyl-3-(4-methoxy-benzoyl)-5-acetamidobenzofuran (compound 2) into a 250ml flask, add 30ml of methanol Make it dissolve, then add 100ml of 15% hydrochloric acid, heat to reflux for 6h, cool, evaporate methanol under low pressure, cool in an ice-water bath, filter to obtain about 4.4g of white solid (compound 3), and then directly purify it without purification After drying, put it into a 100ml flask, add 40ml of toluene and 0.33g (3mmol) of tetramethylammonium chloride, heat to reflux, slowly add 2.8g (24.5mmol) of methanesulfonyl chloride dropwise under reflux, and continue to Reflux for 1h. Cool the reaction, add 40ml of ethyl acetate and 50ml of water solution, wash the organic phase with water (100ml*3), dry over anhydrous sodium sulfate, filter to remove the organic solvent, and obtain 4.4g of white solid (compound 4), two-ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com