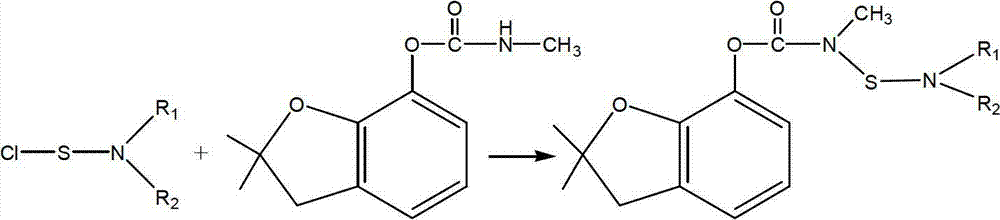
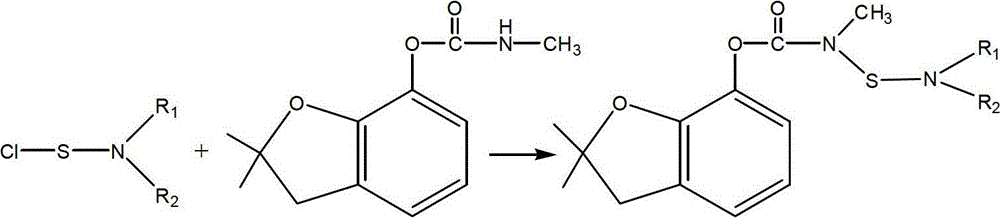
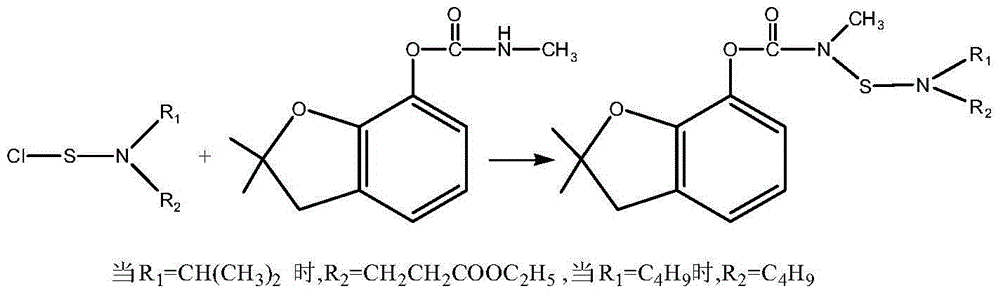
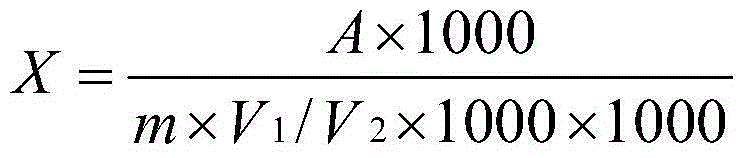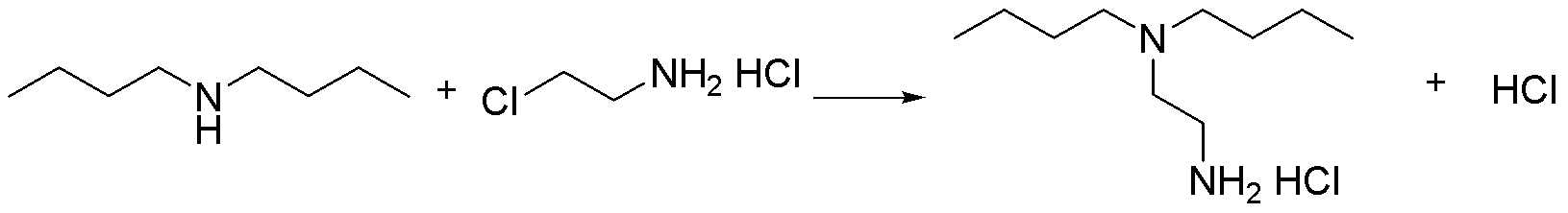Patents
Literature
71 results about "Di-n-butylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Morpholine-substituted poly(arylene ether) and method for the preparation thereof
InactiveUS20110003962A1Improve compatibilityStrong bias toward incorporationAluminium compoundsPlastic/resin/waxes insulatorsPolymer scienceMorpholine
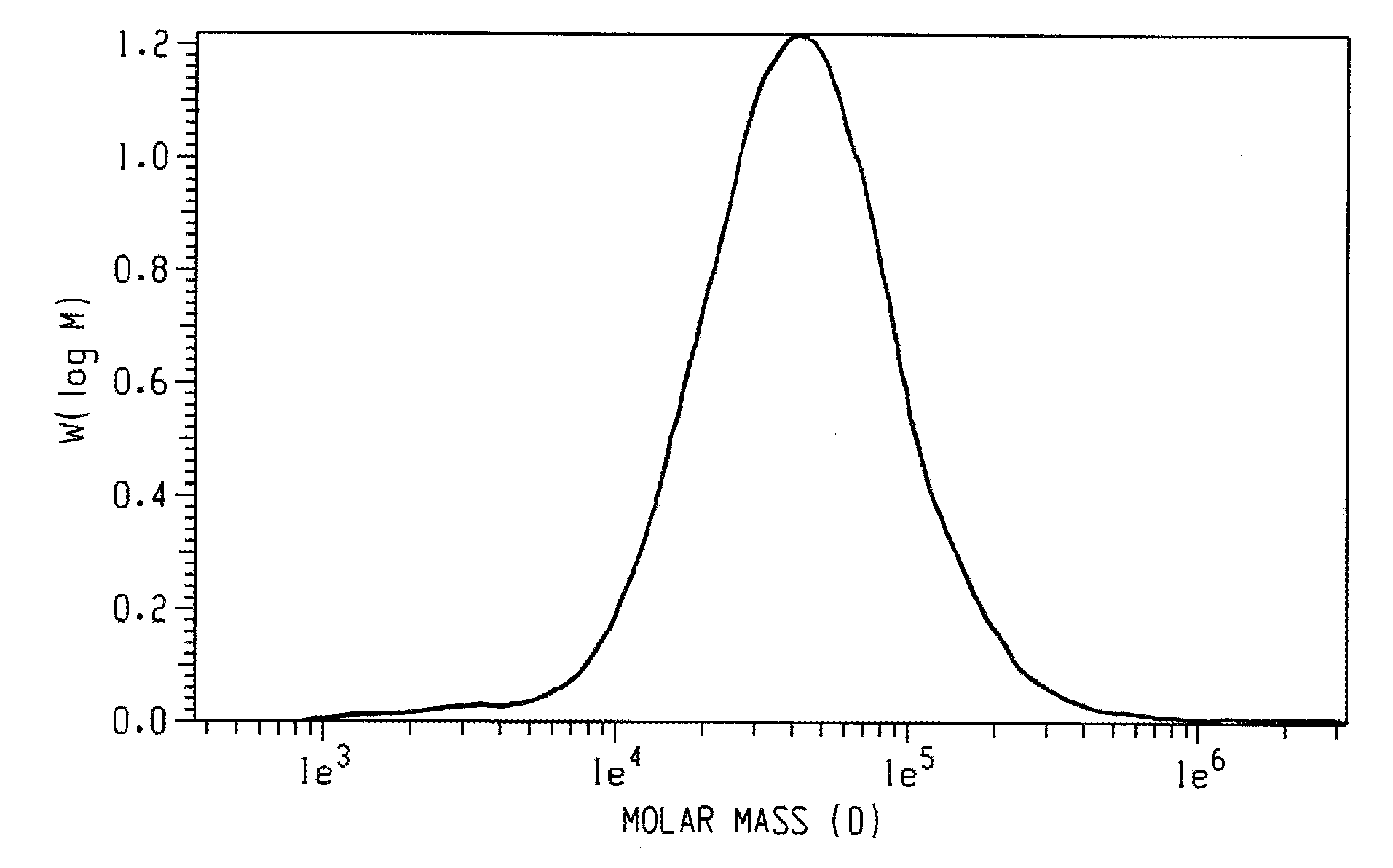
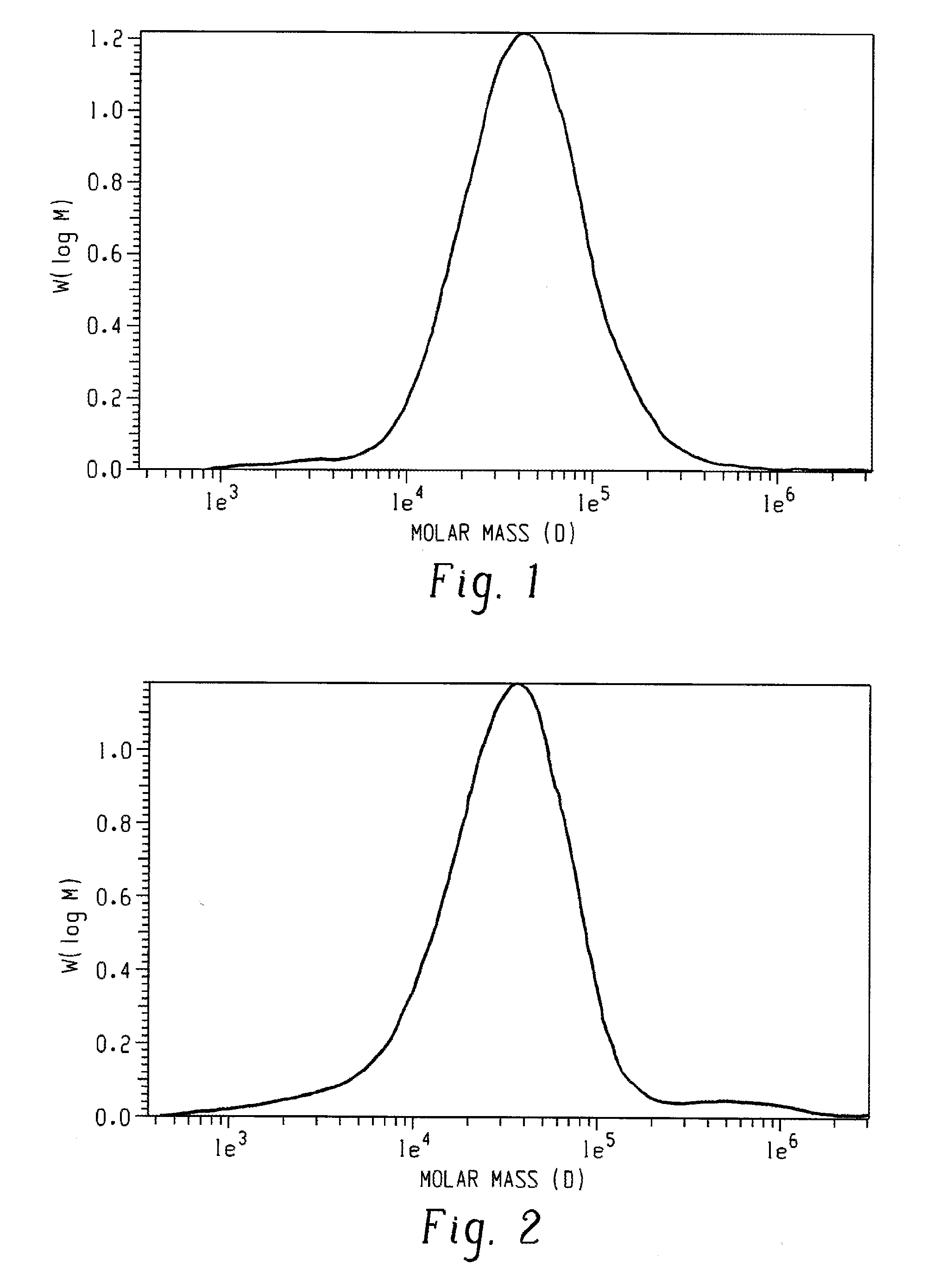
A poly(2,6-dimethyl-1,4-phenylene ether) prepared using a morpholine-containing polymerization catalyst has a monomodal molecular weight distribution with a reduced content of very high molecular weight species. It also exhibits increased morpholine incorporation in the high molecular weight fraction. Compared to commercially available poly(2,6-dimethyl-1,4-phenylene ether) prepared using a di-n-butylamine-containing polymerization catalyst, the poly(2,6-dimethyl-1,4-phenylene ether) of the invention exhibits reduced odor. Compared to other poly(2,6-dimethyl-1,4-phenylene ether) prepared using a morpholine-containing polymerization catalyst, the poly(2,6-dimethyl-1,4-phenylene ether) of the invention exhibits improved molecular weight build during compounding and improved compatibilization with polyamides.
Owner:SABIC GLOBAL TECH BV
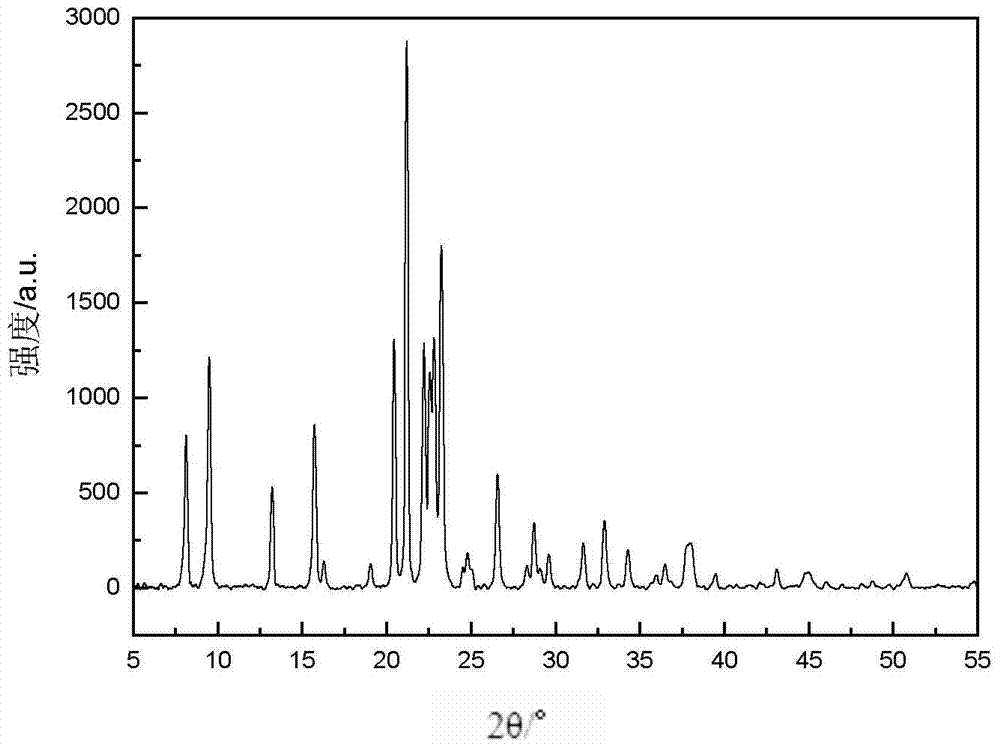
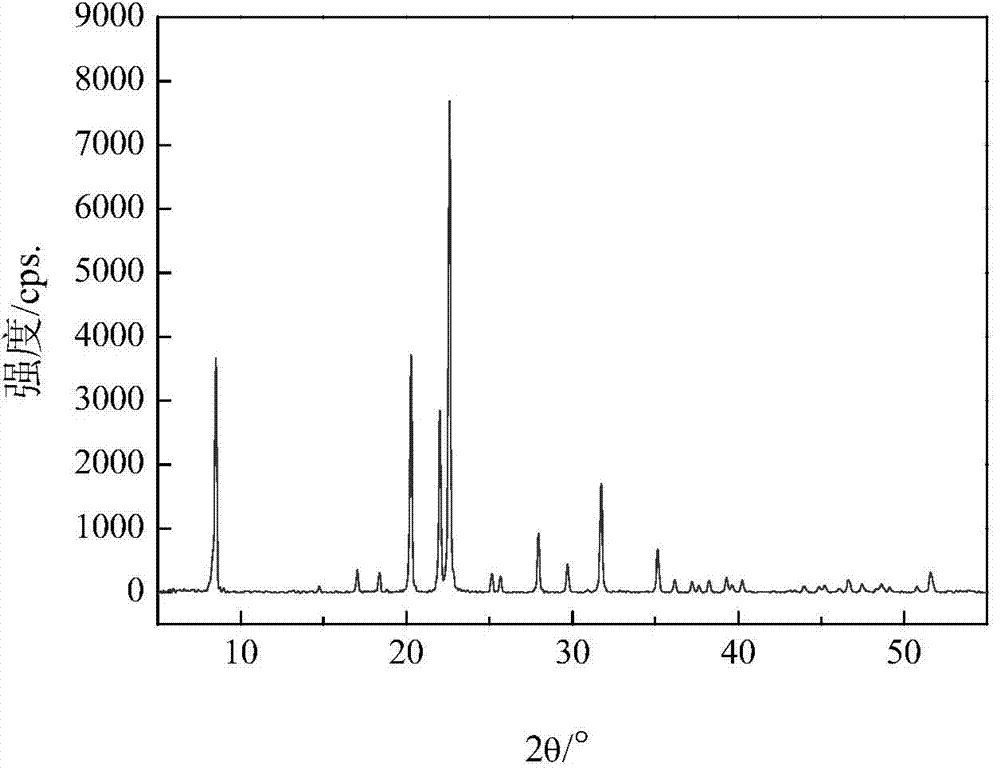
Method for synthesizing SAPO-31 molecular sieves through microwave heating
ActiveCN101786640AAcidity is highly adjustableShorten crystallization timeMolecular sieve catalystsMolecular-sieve and base-exchange phosphatesPhosphorous acidPhosphoric acid
The invention provides a method for synthesizing an SAPO-31 molecular sieve through microwave heating, which relates to a preparation method of an SAPO-31 molecular sieve. The invention solves the problems that the crystallization time of the existing preparation method of the SAPO-31 molecular sieve is long, stray crystals exist in products, and the price of template agents is high. The method of the invention comprises the following steps: firstly, preparing phosphorous acid, water, di-n-butylamine, aluminium isopropoxide and silicon aerosol into gel; then, placing the gel into a reaction kettle to be heated by microwaves; carrying out crystallization at 130 DEG C to 190 DEG C; and then, obtaining the SAPO-31 molecular sieve through centrifugal filtration, washing, drying and roasting. The invention has the advantages of short crystallization time, no stray crystal and low price of the template agents. The SAPO-31 molecular sieve of the invention is applied to the fields of petrochemical industry and fine chemistry industry as a catalyst.
Owner:HEILONGJIANG UNIV
Method for synthesizing SAPO-41 (Phosphoric Acid Silicon-Aluminum-41) molecular sieve by adopting novel template agent
ActiveCN103011196ALow priceReduce dosageMolecular-sieve and base-exchange phosphatesMolecular-sieve silicoaluminophosphatesMolecular sieveSynthesis methods
The invention discloses a method for synthesizing SAPO-41 (Silico-Alumino-Phosphate-41) molecular sieve by adopting a novel template agent, relates to a synthesis method of the silico-alumino-phosphate molecular sieve, in particular relates to a synthesis method of the silico-alumino-phosphate molecular sieve SAPO-41, and solves the problems that the method for synthesizing the SAPO-41 molecular sieve by using a conventional DPA (Di-n-Propylamine) as the template agent is high in preparation cost, has mixed crystal and is not beneficial for the realization of large-scale industrial production. The method comprises the following steps: firstly, preparing 85 percent by mass of phosphoric acid, pseudo-boehmite, di-n-butylamine, silica sol and distilled water into initial gel; and secondly, crystallizing, cooling to the room temperature, centrifuging, washing, drying, roasting and naturally cooling the initial gel to the room temperature so as to obtain the SAPO-41 molecular sieve. The method has the advantages that firstly, the product is in pure phase; secondly, the synthesis cost of the molecular sieve is greatly reduced; and thirdly, the large-scale industrial production is realized. The method is mainly used for synthesizing the SAPO-41 molecular sieve.
Owner:HEILONGJIANG UNIV
High-yield high-purity DAST source powder synthetic process
The invention discloses a high-yield high-purity DAST source powder synthetic process. The synthetic process comprises the following two steps: 1, reacting 4-methylpyridine with methyl p-toluenesulfonate in the presence of absolute ethyl alcohol serving as a solvent to obtain an absolute ethyl alcohol solution of 4-methyl-N-methylpyridine tosilate; and 2, reacting 4-methyl-N-methylpyridine tosilate with p-dimethylaminobenzaldehyde in the presence of absolute ethyl alcohol serving as a solvent and under the catalytic action of di-n-butylamine or piperidine, so as to obtain high-yield (85-95%) high-quality (90-95%) DAST source powder. According to the synthetic process, absolute ethyl alcohol is used as a reaction solvent, harm of poisonous and harmful solvents such as methylbenzene and methyl alcohol to the body of an operator can be avoided and the pollution of waste liquid to the environment can be avoided. The research success of the high-yield high-purity DAST source powder synthetic process is beneficial to culture of high-quality large-sized DAST crystal, thereby laying a good material and theoretical basis for research on the DAST crystal and related products.
Owner:CHINA ELECTRONICS TECH GRP NO 46 RES INST
Preparation process of water-based fluorescent polyurethane compound
ActiveCN105693985AExcellent fluorescence performanceSmall particle sizeLuminescent compositionsWater basedQuantum yield
The invention discloses a preparation process of a water-based fluorescent polyurethane compound. The preparation process includes the following steps that all components are dehydrated for use; dehydrated polypropylene glycol 2000 and dimethylolpropionic acid are added into a three-mouth flask and heated under protection of nitrogen gas, toluene diisocynate and acetone are dropwise added, then dibutyltin dilaurate is added, a reaction is conducted, and in the reaction process, back titration is conducted with di-n-butylamine; the temperature is lowered to 40-50 DEG C, triethylamine is added, and a reaction is conducted; the temperature is lowered to room temperature, triethylamine and ethidene diamine are added, emulsification and reduced pressure distillation are conducted, and the water-based fluorescent polyurethane compound is obtained. The prepared water-based fluorescent polyurethane compound is high in solid content, high in storage stability, high in fluorescence quantum yield and excellent in fluorescence property.
Owner:山东画漫天装饰有限公司
4,4'-methylene bis(dialkyl dithioformamide) preparation method
The present invention relates to a 4,4'-methylene bis(dialkyl dithioformamide) preparation method, which comprises: (1) adding di-n-butylamine to a mixed solution of carbon disulfide and sodium hydroxide in a dropwise manner, maintaining the temperature between 0-30 DEG C, heating to a temperature of 40-60 DEG C after completing the adding of the di-n-butylamine, and continuously reacting for 1-6 h; and (2) adding methylene chloride to the obtained solution in a dropwise manner, heating to a temperature of 50-90 DEG C after completing the adding of the methylene chloride, reacting for 0.5-8 h, and carrying out separation purification to obtain the product. With the method of the present invention, the feeding way of the material is changed, the high risk level carbon disulfide is not required to be added in the dropwise manner, other solvents are not required to be added, the reaction time and the operation steps are saved, the yield is significantly improved, and the product performance is excellent.
Owner:CHINA PETROLEUM & CHEM CORP +1
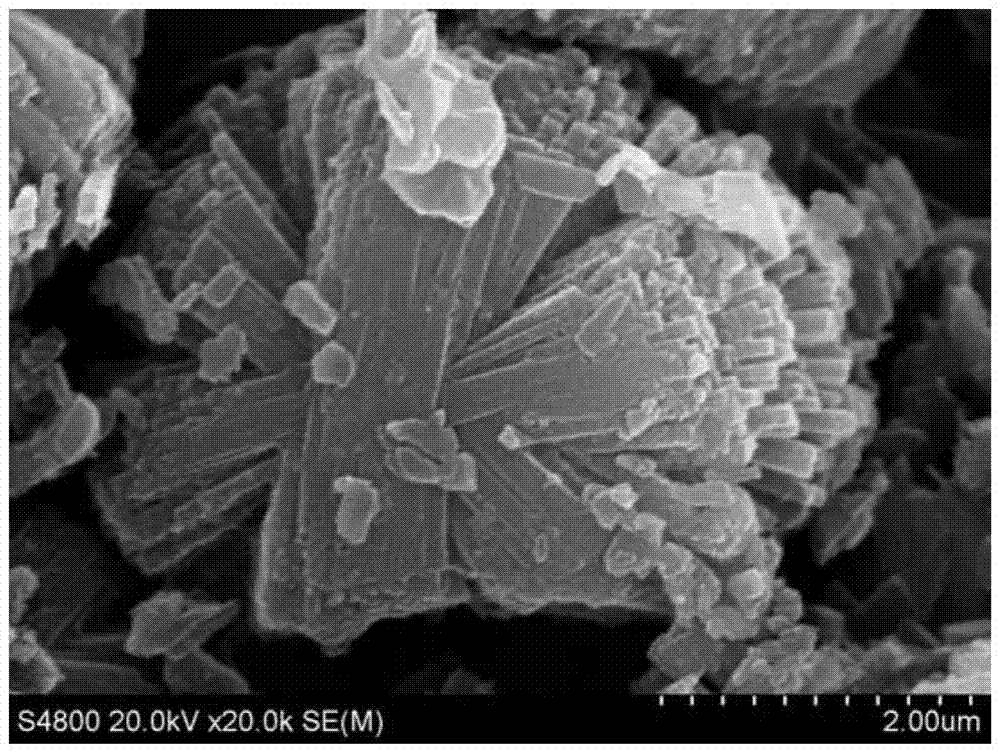
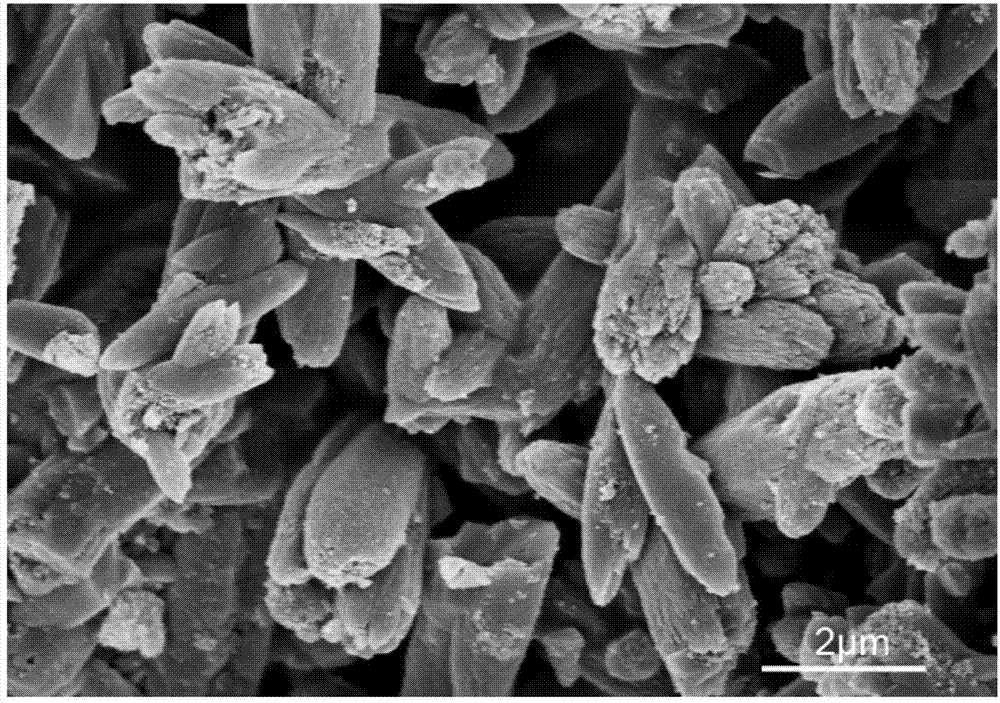
Method for preparing SAPO-11 molecular sieve
ActiveCN103523795AAcidity is highly adjustableMolecular-sieve and base-exchange phosphatesMolecular-sieve silicoaluminophosphatesMolecular sieveCentrifugation
The invention relates to a method for preparing the SAPO molecular sieve to solve the problem that other SAPO class molecular sieve mixed crystals are easily generated through an existing method for preparing the SAPO-11 molecular sieve. The method includes the step of preparing initial gel, namely, firstly, preparing phosphoric acid, pseudo-boehmite, di-n-butylamine, silica sol and deionized water into the initial gel, and the step of crystallization and post-processing, namely, conducting crystallization on the initial gel prepared in the first step, naturally cooling crystallized gel till the crystallized gel is at the room temperature, and conducting centrifugation, washing, drying and calcination to obtain the SAPO-11 molecular sieve. The SAPO-11 molecular sieve prepared through the method is an aggregate formed by regularly arraying pure-phase high-crystallinity stick-shaped crystalline grains, the SAPO-11 molecular sieve with the silica-aluminium ratio ranging from 0.2 to 0.8 can be synthesized through the method, and the adjustability of the acidity of the molecular sieve can be better. The method can be used for preparing the SAPO-11 molecular sieve.
Owner:HEILONGJIANG UNIV
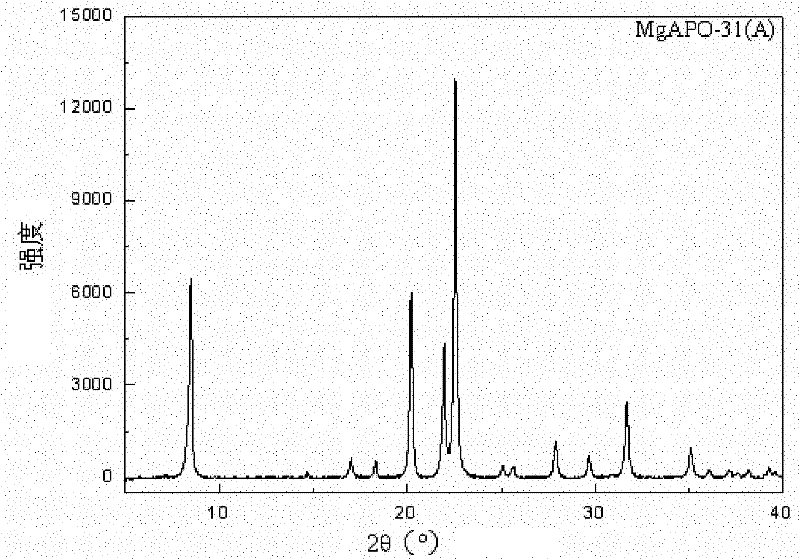
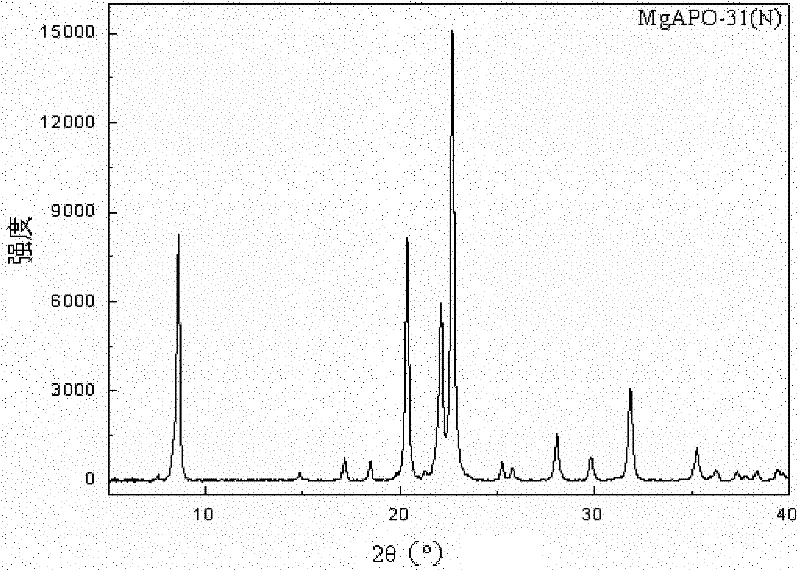
Method for synthesizing heteroatom substituted MeAPO-31 molecular sieve by microwave heating
InactiveCN102001682AShorten crystallization timeLow costMolecular-sieve and base-exchange phosphatesMolecular-sieve silicoaluminophosphatesPhosphoric acidPetrochemical
The invention discloses a method for synthesizing a heteroatom substituted MeAPO-31 molecular sieve by microwave heating, relating to a preparation method of a MeAPO-31 molecular sieve. The invention aims to solve the technical problems that the traditional method for preparing the MeAPO-31 molecular sieve has long crystallization time, easy generation of mixed crystals in products, limited type and quantity of the heteroatom introduced into a frameworkd, the narrow adjustable range of the produced acid sites and the like. The method comprises the following steps of: firstly, preparing gel by using phosphoric acid, water, di-n-butylamine, aluminum isopropoxide (or boehmite) and metal salt; heating with microwaves; and crystallizing, then centrifugally filtering, washing, drying and roasting to obtain the MeAPO-31 molecular sieve. Compared with the traditional hydrothermal process, the method disclosed in the invention obviously shortens the crystallization time of the molecular sieve and does not generate mixed crystals. The MeAPO-31 molecular sieve prepared with the method can be used as catalysts in the fields of petrochemical industry, fine chemical industry and the like.
Owner:HEILONGJIANG UNIV
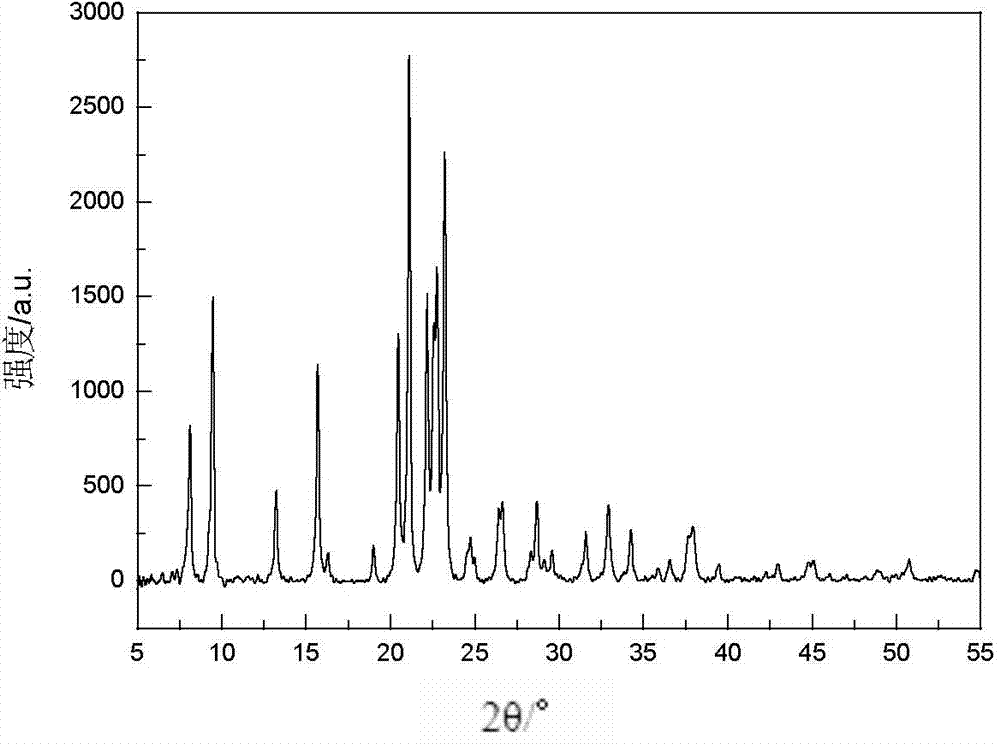
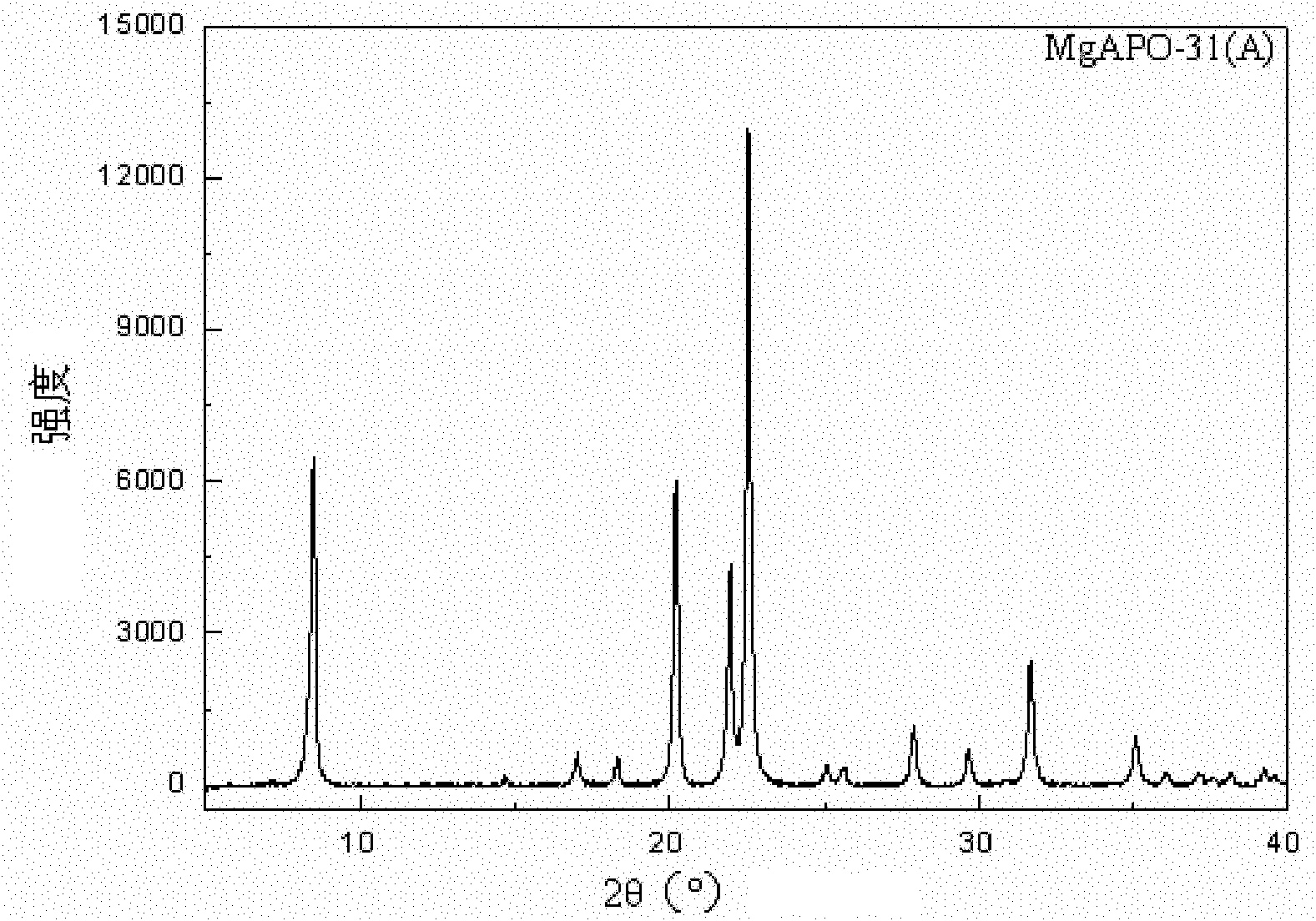
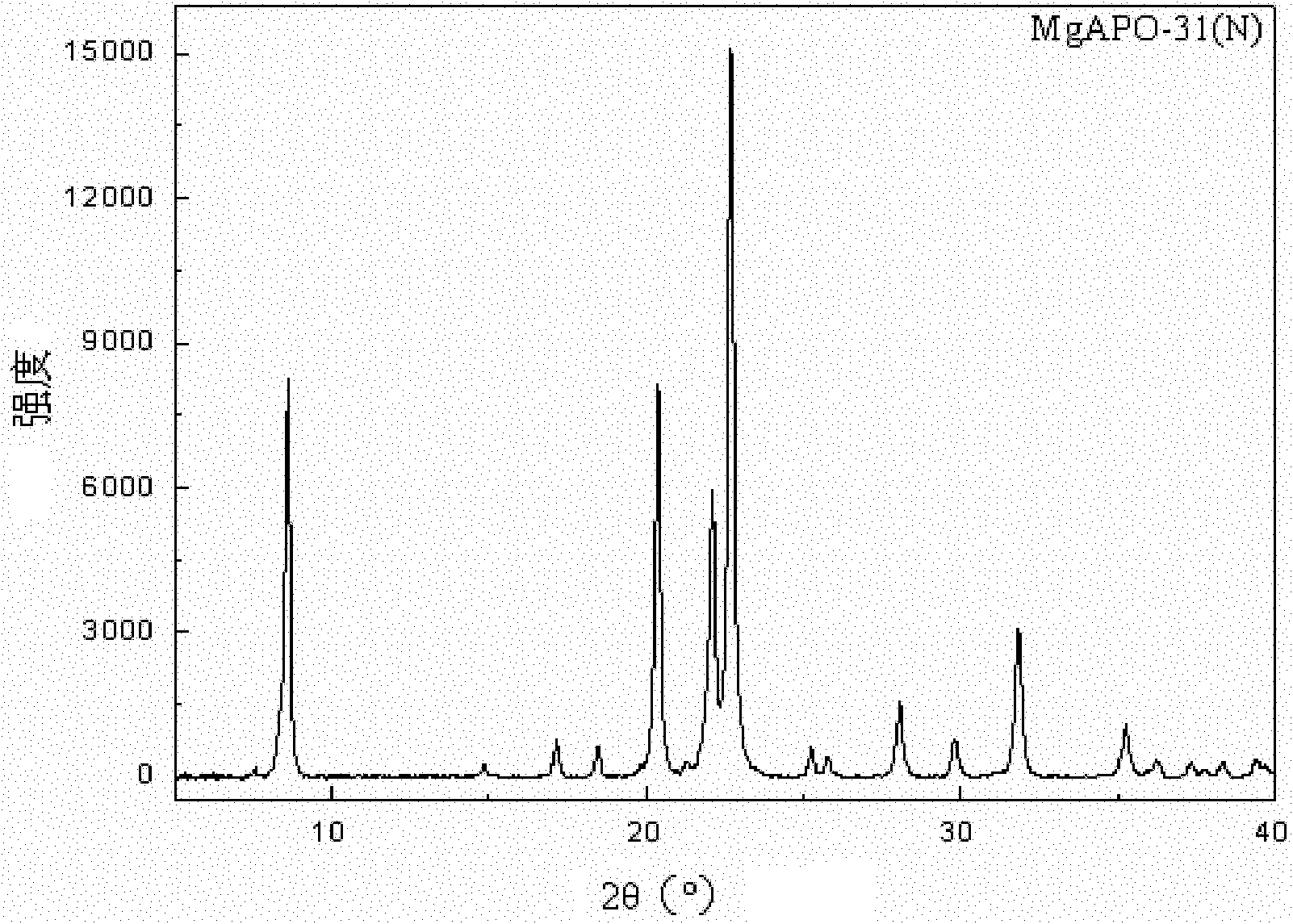
Synthetic method of MgSAPO-31 molecular sieve
InactiveCN103482647AHigh purityHigh densityMolecular-sieve and base-exchange phosphatesMolecular-sieve silicoaluminophosphatesIon exchangePhosphoric acid
The invention discloses a synthetic method of a MgSAPO-31 molecular sieve, relating to a synthetic method of a molecular sieve, and aiming to solve the problems that only a molecular sieve with an acid site can be synthesized at present by adopting the conventional silicon source and aluminum source and mixed crystals are easily formed in a crystallized product. The synthetic method comprises the steps of 1, dispersing a NaX molecular sieve into a magnesium nitrate solution or magnesium acetate solution to perform ion exchange, and controlling the exchange frequency and exchange time to obtain a Mg / NaX molecular sieve; 2, mixing phosphoric acid, pseudo-boehmite, the Mg / NaX molecular sieve, di-n-butylamine and deionized water, and stirring to obtain an initial gel; 3, putting the initial gel into a crystallization kettle, performing crystallization at 170-190 DEG C, performing centrifugal separation, washing and drying on a crystallization product, and then roasting to obtain a molecular sieve. According to the synthetic method disclosed by the invention, a molecular sieve framework contains two acid sites of Si and Mg with different strengths at the same time, the preparation purity is high, and the molecular sieve can be used as a catalyst applied to the fields of petrochemical industry and the like.
Owner:HEILONGJIANG UNIV
Preparation process for tetrabutyl urea
InactiveCN102702029AImprove product qualityRaw materials are cheap and easy to getUrea derivatives preparationOrganic compound preparationOrganic solventCarbonyl chloride
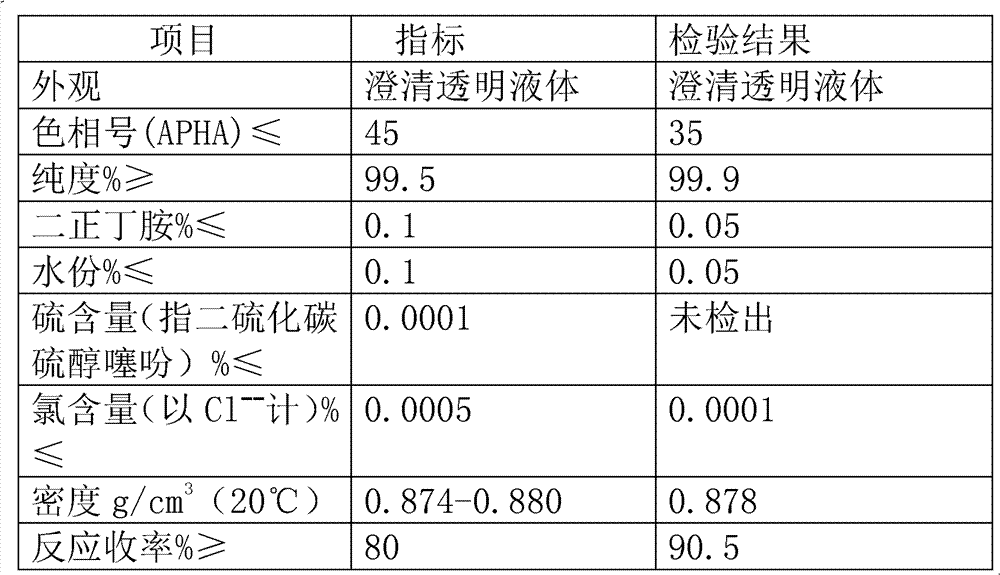
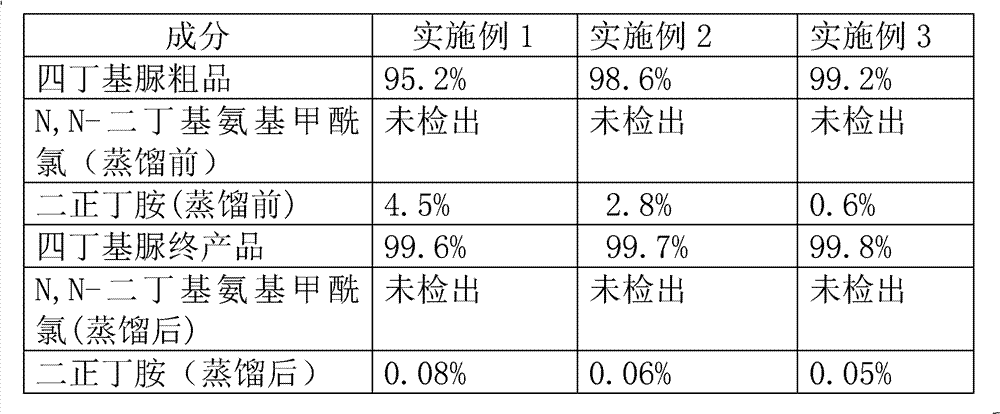
The invention provides a preparation process for synthesizing tetrabutyl urea by using di-n-butylamine and carbonyl chloride as major materials in an alkaline organic solvent by means of phosgenation synthesis, wherein the process comprises the following steps: (1) adding di-n-butylamine, carbonyl chloride and organic solvent to a reactor in certain proportion; (2) adjusting the temperature of acylation reaction and controlling the reaction time to obtain crude tetrabutyl urea; (3) standing, separating, distilling and purifying the crude tetrabutyl urea to obtain a final product of tetrabutyl urea. The preparation method provided by the invention is simple in procedure, the raw materials are cheap and easily available, the product is good in quality, the reaction yield is over 90%, and the purity is more than 99%.
Owner:CHONGQING CHANGFENG CHEM IND
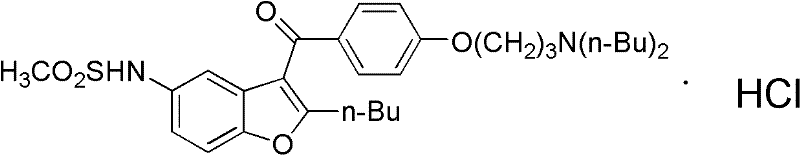
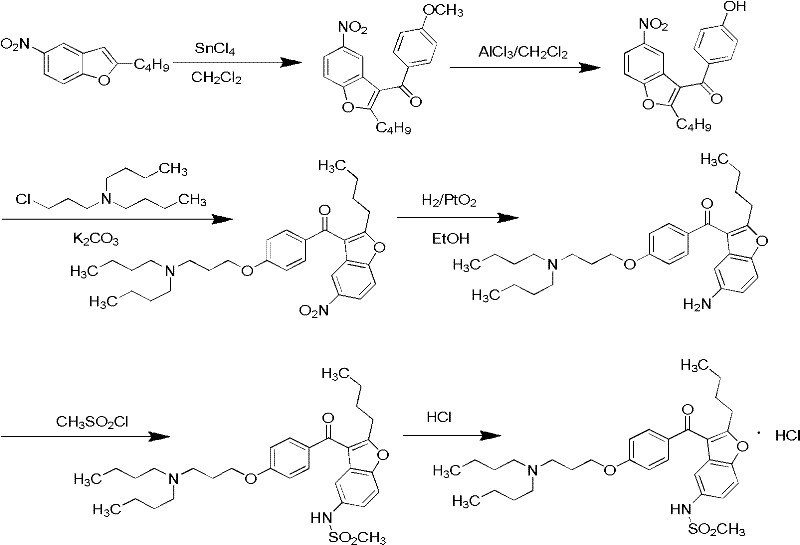
Method for preparing dronedarone hydrochloride
InactiveCN102382087ALess toxicLow process pollutionOrganic chemistrySulfonyl chlorideMethanesulfonyl chloride
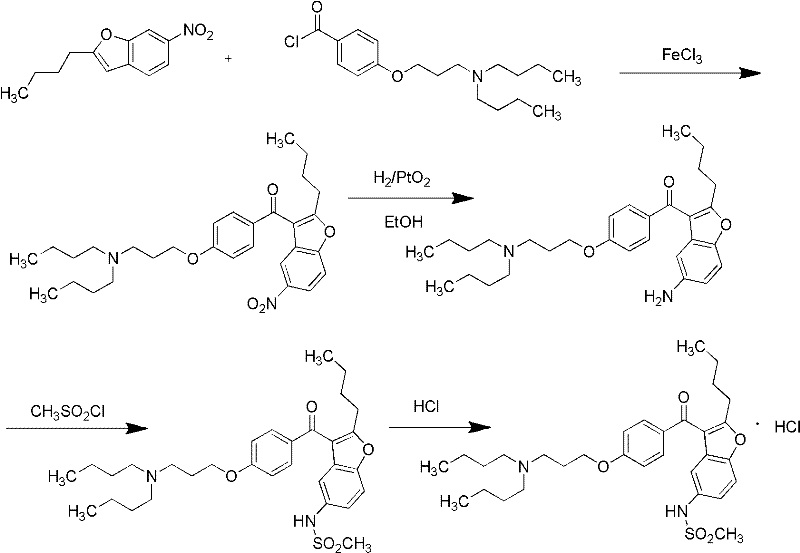
The invention relates to a method for preparing dronedarone hydrochloride, comprising the following concrete steps of: (1) carrying out etherification reaction on 2-butyl-3-(4-hydroxy benzoyl)-5-nitro benzofuran and 1-bromine-3-chloropropane in an organic solvent so as to obtain 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-nitro benzofuran; (2) reducing to 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-amido benzofuran by utilizing a reductant; (3) carrying out sulfamide reaction with methyl sulfonyl chloride in the organic solvent so as to obtain 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-methyl sulfonamide benzofuran; (4) carrying out N-hydrocarbon reaction with di-n-butylamine so as to obtain the dronedarone; and (5) salifying the dronedarone hydrochloride and obtaining the dronedarone hydrochloride. The method provided by the invention has the advantages that the reaction route is simple, the raw material is cheap and easy to obtain and the reaction condition is mild and easy to control and is suitable for industrialized production.
Owner:NANJING UNIV OF TECH
Organic molecular ferroelectric crystal di-n-butylamine difluoromonochloroacetate, preparation method therefor and use of organic molecular ferroelectric crystal di-n-butylamine difluoromonochloroacetate
ActiveCN106480506AImprove stabilitySaturation polarization is highPolycrystalline material growthFrom normal temperature solutionsHysteresisSaturation polarization
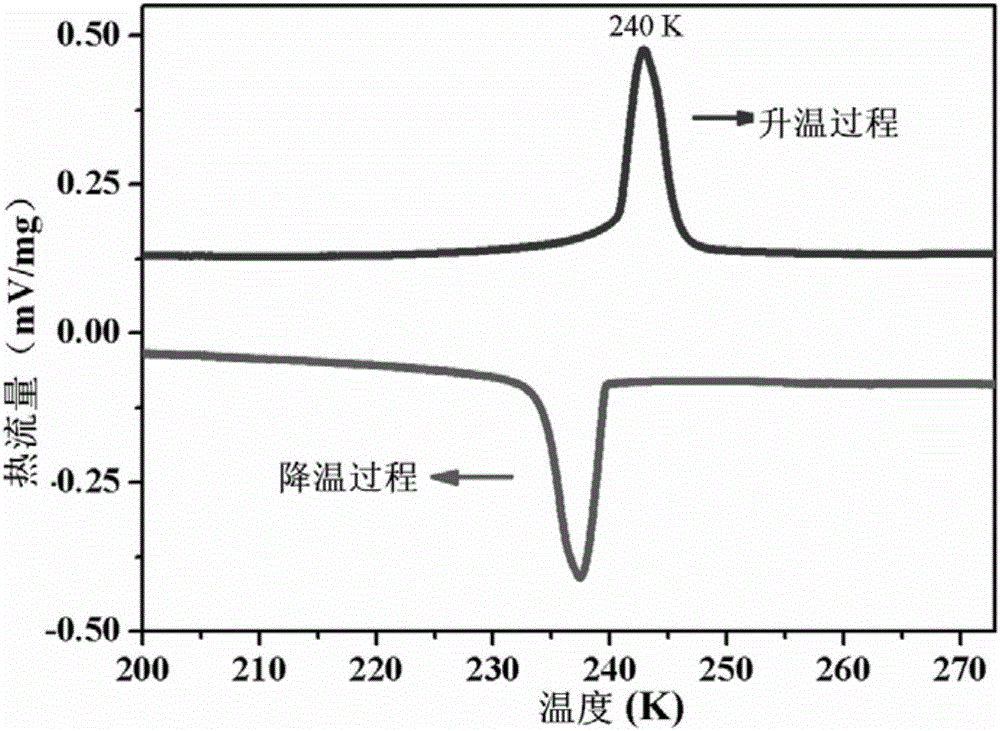
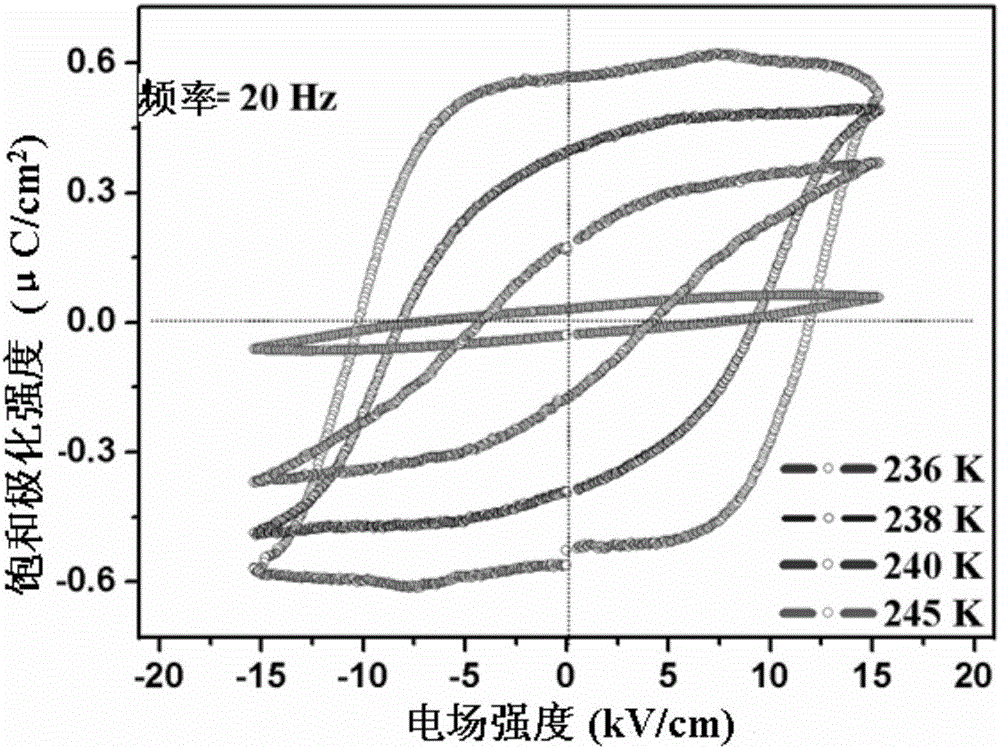
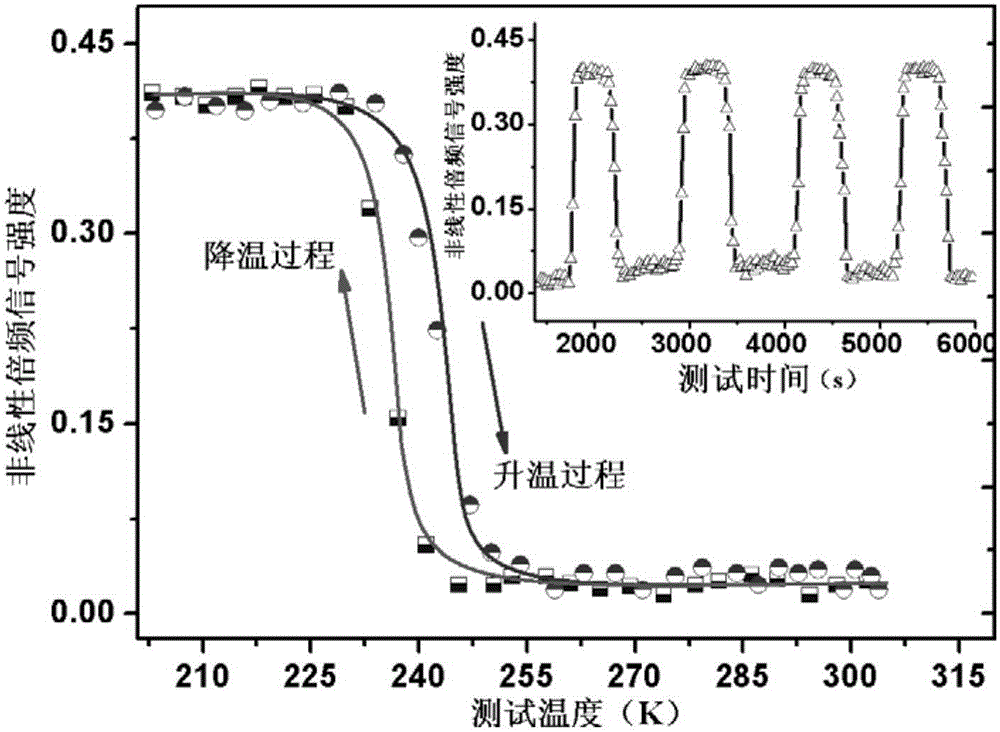
The invention relates to an organic molecular ferroelectric crystal di-n-butylamine difluoromonochloroacetate, a preparation method therefor and use of the organic molecular ferroelectric crystal di-n-butylamine difluoromonochloroacetate. Proven by tests on electric hysteresis loop of the organic molecular ferroelectric crystal material, i.e., di-n-butylamine difluoromonochloroacetate through a Sawyer-Tower circuit, the material shows good ferroelectric properties in a ferroelectric phase and has relatively high saturation polarization strength reaching 3.9[mu]c / cm<2> and moderate coercive field about 12.4kV / cm. Proven by variable temperature nonlinear tests, the material does not have obvious second-order nonlinear frequency-doubled signals in a paraelectric phase, obvious second-order nonlinear frequency-doubled signals appear when the temperature is cooled to Curie temperature (243K), the strength value of the nonlinear signals is saturated along with decrease of temperature, excellent nonlinear on / off performance is shown in an entire test temperature range, the on / off ratio reaches 28, and the repeatability is relatively good. The reaction is simple, and the conditions are moderate.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
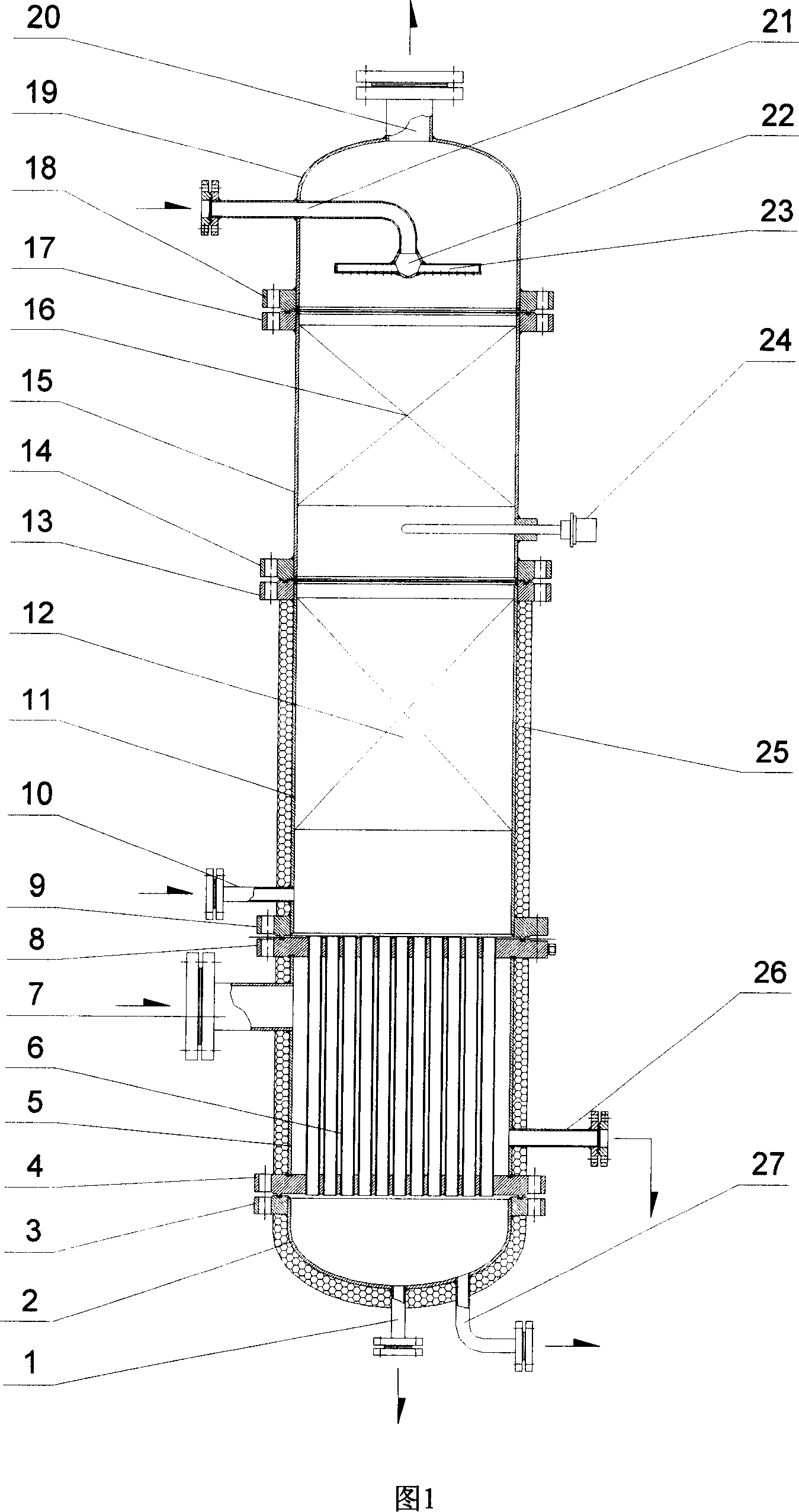
Hot reflux tower for producing carbon-13
InactiveCN101053815AReduce consumptionReduce manufacturing costIsotope separationChemical/physical/physico-chemical processesChemical reactionDecomposition
A hot reflux tower for producing carbon-13 belongs to a chemical reaction device art. The invention discloses a hot reflux tower for producing carbon-13, which mainly comprises the following five parts from above to below: a tower top cover with a calandria liquid distributors, a direct heat exchange-cold liquid leaching tower section filling with structured packing, a decoposing tower section, a cellpacking standing heater tower pot and a tower bottom cap, wherein the heat travels regularly in a shell pass, and the working material runs along a pipe pass. Decomposition products of the hot reflux are carbon dioxide and solution of di-n-butylamine / octane which flows out from the bottom of the tower pot, and the carbon dioxide flows out from the top of the tower top cover. The invention replaces an expensive cellpacking standing gas cooler of the existing technology by the direct heat exchange-cold liquid leaching tower section filling with structured packing, and is provided with a temperature control component for controlling decomposition temperature at 60 + / -5 DEG C., which precision of the control temperature is not + / - 1 DEG C. but + / - 5 DEG C. Accordingly, energy consumption is saved about 20%, in addition to saving the investment cost, and crystallization accident is avoided.
Owner:杨国华
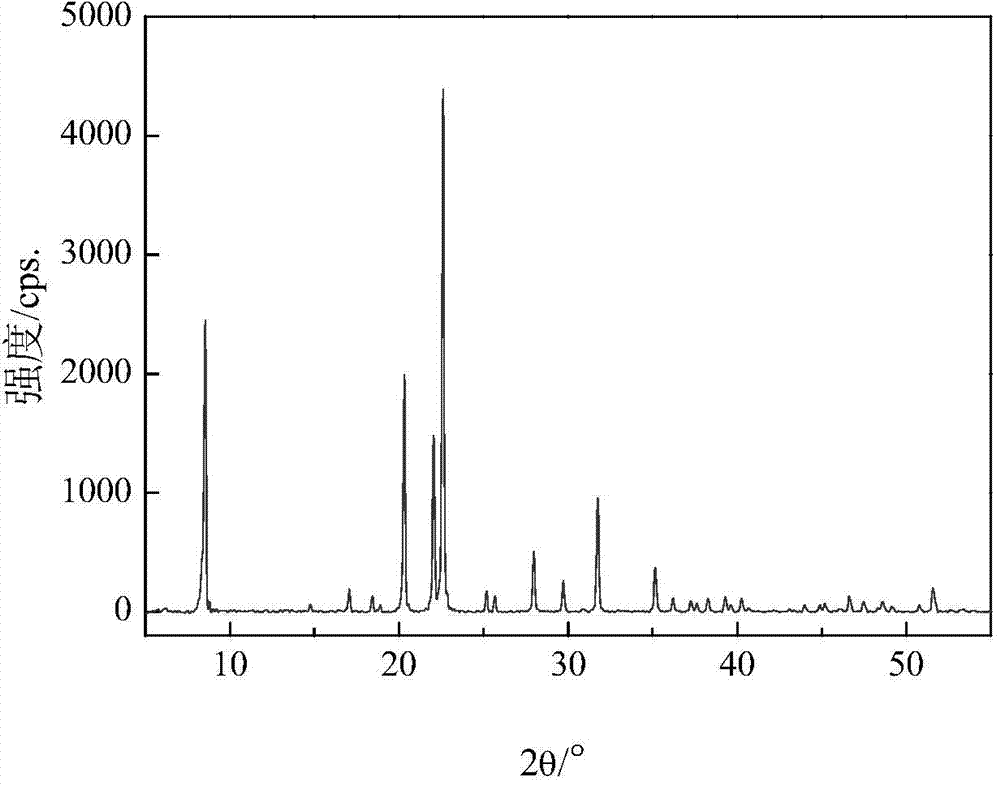
SAPO-31 molecular sieve and preparation method thereof
ActiveCN102992350ALow costEasy to makeMolecular-sieve and base-exchange phosphatesMolecular-sieve silicoaluminophosphatesMolecular sievePhosphoric acid
An SAPO-31 molecular sieve and a preparation method thereof relate to a molecular sieve and a preparation method thereof. The invention aims to solve problems in an existing preparation method of the SAPO-31 molecular sieve, such as long crystallization time, low yield, complex operation, unsuitability for mass production, high cost of the prepared SAPO-31, and easy generation of SAPO-5 mixed crystal, SAPO-11 mixed crystal and SiO2 mixed crystal. The SAPO-31molecular sieve is prepared from phosphoric acid, pseudoboehmite, silica sol and di-n-butylamine. The preparation method comprises steps of: 1. initial gel preparation; 2. crystallization; and 3. roasting to obtain the SAP-O31 molecular sieve. The invention is applicable to the fields of catalyst and petrochemical processing.
Owner:HEILONGJIANG UNIV
Preparation process of tetrabutyl urea
InactiveCN107868022AImprove product qualityRaw materials are cheap and easy to getUrea derivatives preparationOrganic compound preparationOrganic solventReaction rate
The invention provides a preparation process for synthesizing tetrabutylurea by using the phosgene method, using di-n-butylamine and phosgene as the main ingredients in an alkaline organic solvent, and the process steps are as follows: (1) in a reaction vessel according to the composition than adding di-n-butylamine, carbonyl chloride and an organic solvent; (2) adjust the acylation reaction temperature, control the acylation reaction time, and obtain the crude product of tetrabutylurea; (3) the crude product of tetrabutylurea is subjected to standing, separation, Distillation and purification to obtain the final product of tetrabutylurea. The preparation method of the invention has simple and convenient operation steps, cheap and easy-to-obtain raw materials, good product quality, a reaction yield of more than 90 percent, and a purity of more than 99 percent.
Owner:青岛九洲千和机械有限公司
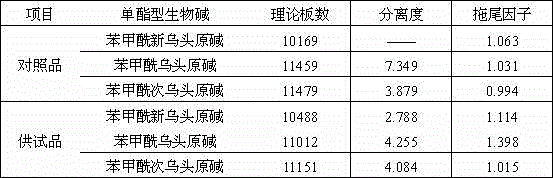
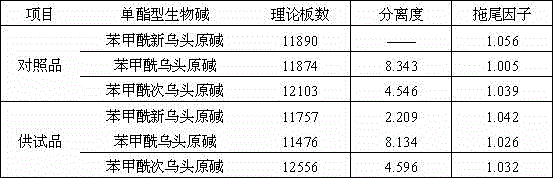
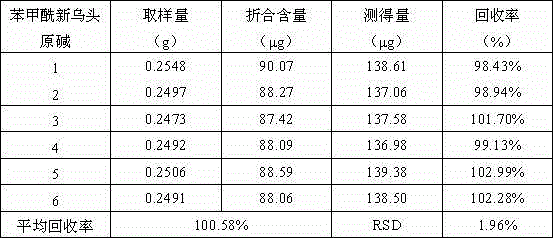
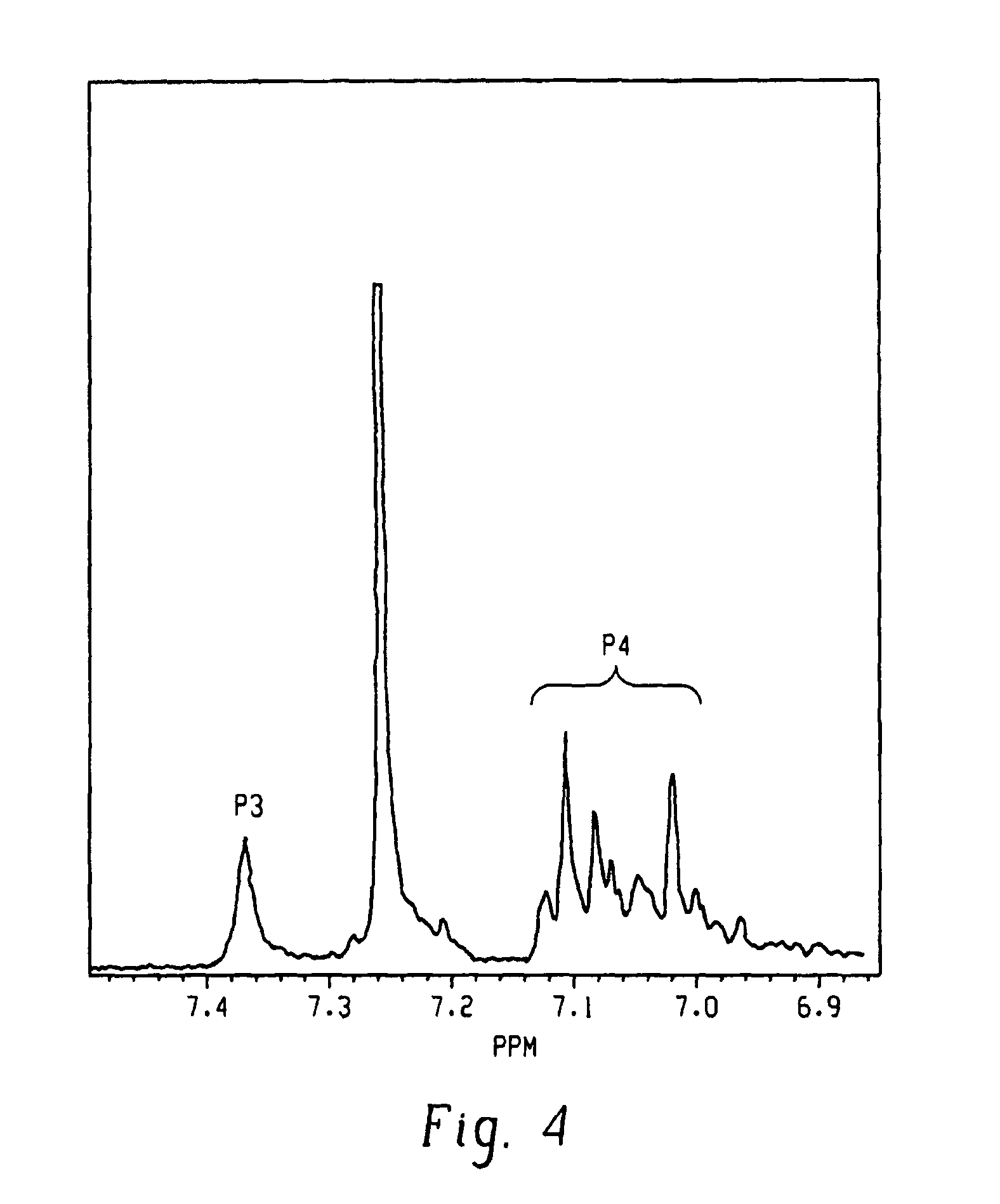
Method for determining content of mono-ester aconitum alkaloids in ephedra-aconitum carmichaelii-liquorice preparation
The invention provides a method for determining the content of mono-ester aconitum alkaloids in an ephedra-aconitum carmichaelii-liquorice preparation. According to the method, high performance liquid chromatography is used for determining the content of mono-ester aconitum alkaloids, a chromatographic column taking polar ether connected phenyl bonded silica gel as filler, and acetonitrile and 0.05-0.2% phosphoric acid solution which are in a ratio of (19:81)-(25:75) are taken as flowing phases, wherein the phosphoric acid solution comprises 0.02%-0.06% of triethylamine and 0.01-0.03% of di-n-butylamine, the detection wavelength is 222-242nm, the number of theoretical plates is not lower than 6000 if calculated by benzoyl mesaconine peak. The separation degree of target components, peak forms and tailing factors are all improved greatly, and moreover, the specificity, linearity, accuracy, precision and durability are all good.
Owner:SHANDONG TIANSHUN PHARMA
Preparation method for sulfur-containing carbofuran derivative with less than 0.1% of harmful impurity carbofuran
ActiveCN102786503ATroubleshoot incomplete responsesSolve the technical problem of high carbofuran contentOrganic chemistryCarbofuranEthane Dichloride
The invention discloses a preparation method for sulfur-containing carbofuran derivative with less than 0.1% of harmful impurity carbofuran. The preparation method comprises the following steps of: using di-n-butylamine or N-isopropyl-beta-alanine ethyl ester derivative and sulfur monochloride as raw materials to prepare into disulphide in the presence of an acid-binding agent triethylamine and an organic solvent 1, 2-dichloroethane; and then reacting with a chlorinating agent sulfuryl chloride to obtain nitrogen sulfur chlorine; and finally reacting with carbofuran under the effect of a cosolvent N-methyl-2-pyrrolidone or dimethylformamide so as to obtain the sulfur-containing carbofuran derivative with not less than 96% of quality percentage composition and less than 0.1% of main harmful impurity carbofuran. By adopting the preparation method, the problem that the carbofuran cannot be completely reacted during being reacted can be solved, the defects due to low content and low yield of sulfur-containing carbofuran derivatives such as benfuracarb and carbosulfan caused by a solid-liquid two-phase reaction can be avoided, the technical problem due to relatively high content of main harmful impurity carbofuran can be solved, and the content of the sulfur-containing carbofuran derivatives such as benfuracarb and carbosulfan can reach a value not less than 96%, and the yield is 97 to 98%, and the main harmful carbofuran in the product is less than or equal to 0.1%, so that the preparation method has a wide popularization and application prospect.
Owner:湖南海利常德农药化工有限公司
Preparation method of dialkyldithiocarbamate
The invention provides a preparation method of dialkyldithiocarbamate. The preparation method includes following steps: mixing sodium hydroxide, water, di-n-butylamine, alcohol, dichloromethane and carbon disulfide for reaction to obtain a reaction product; adopting solvent oil to extract the reaction product, and separating to obtain an oil phase; mixing the oil phase with a supporter, and filtering to obtain mother liquid; performing depressurizing distillation on the mother liquid to obtain distilled mother liquid; adopting the solvent oil to extract the distilled mother liquid, and performing depressurizing distillation to obtain dialkyldithiocarbamate. The preparation method prepares dialkyldithiocarbamate through a one-step process, the objective of no washing with water is achieved through two times of extraction through the solvent oil, two times of depressurizing distillation and treatment by adopting the supporter before a time of depressurizing distillation, so that generation of wastewater is reduced, and high product yield and product purity are realized. Experiment results show that yield of dialkyldithiocarbamate is higher than 99%, and purity is higher than 98%.
Owner:XINXIANG RICHFUL LUBE ADDITIVE CO LTD
Preparation method for sulfur-containing carbofuran derivative with less than 0.1% of harmful impurity carbofuran
ActiveCN102786503BTroubleshoot incomplete responsesSolve the technical problem of high carbofuran contentOrganic chemistryCarbofuranEthane Dichloride
The invention discloses a preparation method for sulfur-containing carbofuran derivative with less than 0.1% of harmful impurity carbofuran. The preparation method comprises the following steps of: using di-n-butylamine or N-isopropyl-beta-alanine ethyl ester derivative and sulfur monochloride as raw materials to prepare into disulphide in the presence of an acid-binding agent triethylamine and an organic solvent 1, 2-dichloroethane; and then reacting with a chlorinating agent sulfuryl chloride to obtain nitrogen sulfur chlorine; and finally reacting with carbofuran under the effect of a cosolvent N-methyl-2-pyrrolidone or dimethylformamide so as to obtain the sulfur-containing carbofuran derivative with not less than 96% of quality percentage composition and less than 0.1% of main harmful impurity carbofuran. By adopting the preparation method, the problem that the carbofuran cannot be completely reacted during being reacted can be solved, the defects due to low content and low yield of sulfur-containing carbofuran derivatives such as benfuracarb and carbosulfan caused by a solid-liquid two-phase reaction can be avoided, the technical problem due to relatively high content of main harmful impurity carbofuran can be solved, and the content of the sulfur-containing carbofuran derivatives such as benfuracarb and carbosulfan can reach a value not less than 96%, and the yield is 97 to 98%, and the main harmful carbofuran in the product is less than or equal to 0.1%, so that the preparation method has a wide popularization and application prospect.
Owner:湖南海利常德农药化工有限公司
Method for preparing photo-crosslinking polyurethane film
The invention relates to the field of polyurethane and particularly relates to a method for preparing a photo-crosslinking polyurethane film. The method comprises the following steps: adding 24.5g of isophorone diisocyanate and 4 droplets of catalyst into a 250ml four-mouthed bottle, adding 57.1g of polyether polyol into a dropping funnel, carrying out dropwise adding, carrying out a reaction for 1 to 1.5 hours while controlling the temperature to 40 DEG C to 50 DEG C, measuring NCO content by a di-n-butylamine method, adding 3.75g of 2,2-bishydroxymethyl propionate after the NCO content meets requirements, carrying out a reaction for 2 to 3 hours at the reaction temperature of 60 DEG C to 70 DEG C, dropwise adding 3.47g of hydroxyl-2-ethyl methacrylate and a polymerization inhibitor when the NCO content is approximate to a theoretical value, heating the temperature to 70 DEG C, carrying out a reaction for 3 to 4 hours, carrying out sampling, carrying out infrared detection, and obtaining a polyurethane prepolymer when characteristic peaks of an NCO group disappear; loading the prepolymer into a four-mouthed flask, adding triethylamine into the four-mouthed flask according to the mole ratio of 1: 1 in a dropwise adding manner, and carrying out a neutralization reaction for 0.5 to 1 hour at the temperature of 50 DEG C, thereby obtaining a composite nano-emulsion. According to the method, the operation is simple, the operation is convenient, the comprehensive performance of the product is good, and the method is efficient, energy-saving, environmentally friendly, safe and sanitary.
Owner:SHAANXI QIYUAN TECH DEV
Intermediate and method used for preparing dronedarone
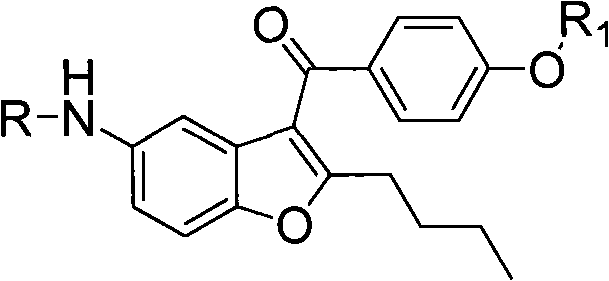
InactiveCN102532074AAvoid hydrogenation catalysisRaw materials are easy to getOrganic chemistryBulk chemical productionAnisoyl chlorideDronedarone
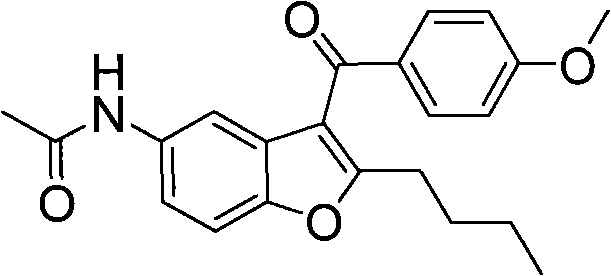
The invention relates to an intermediate and method used for preparing dronedarone and belongs to the technical field of cardiovascular drugs. The method comprises the following steps of: using 2-n-butyl-5-acetamidobenzobfuran to react with p-anisoyl chloride under the catalysis of Lewis acid and obtain 2-n-butyl-3-(4-methoxy-benzoyl)-5-acetamidobenzobfuran; acidizing to obtain 2-n-butyl-3-(4-methoxy-benzoyl)-5-benzofuranamin hydrochloride, reacting with methylsulfonyl chloride to obtain 2-n-butyl-3-(4-methoxy-benzoyl)-5-methylsulfonylamidobenzobfuran; obtaining 2-n-butyl-3-(4-hydroxyl-benzoyl)-5-methylsulfonylamidobenzobfuran under the action of the Lewis acid; and reacting with 1,3-dibromopropane to obtain 2-n-butyl-3-[4-(gamma-bromopropyl)hydroxyl-benzoyl]-5-methylsulfonylamidobenzobfuran, and then reacting with di-n-butylamine to obtain dronedarone. The method avoids the catalytic hydrogenation reaction and has the advantages of available raw materials, simple operation process, high yield, easiness in industrialization and the like.
Owner:TIANJIN KELIN CHEM
Method for determining synthetical coloring agent in food
The invention discloses a method for determining a synthetical coloring agent in food, which is short in detecting time, and is high in working efficiency. According to the technical scheme, the method comprises the following steps: putting 2g of homogenized sample into a centrifugal pipe of 50ml, adding 10ml of water, performing ultrasonic treatment for 5 minutes, adding 2ml of hydrochloric acid, shaking evenly, then adding 10ml of 5% di-n-butylamine n-butyl alcohol solution, whirling for 1 minute, centrifuging at 4,000r / min for 3 minutes, absorbing 2ml of supernate to another centrifugal pipe, adding about 10ml of n-hexane, shaking evenly, then adding 2.0ml of 40% ammonia water solution, vibrating and shaking forcefully, whirling for 1 minute, standing for layering, adding 1.0ml of lower solution into a centrifugal pipe of 2ml, then adding 0.50ml of glacial acetic acid again, shaking evenly, centrifuging at 15,000r / min for 5 minutes, and putting 10 micro-liter of obtained solution into a high performance liquid chromatograph. The method belongs to the field of food detecting techniques.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
Low-temperature cured epoxy resin paint and preparation method thereof
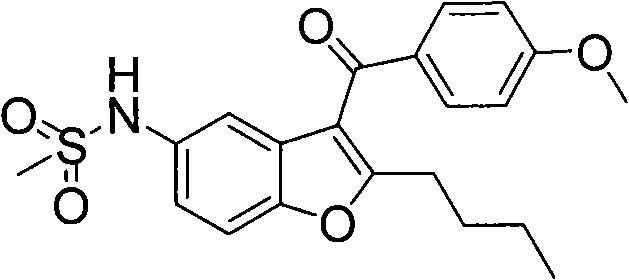
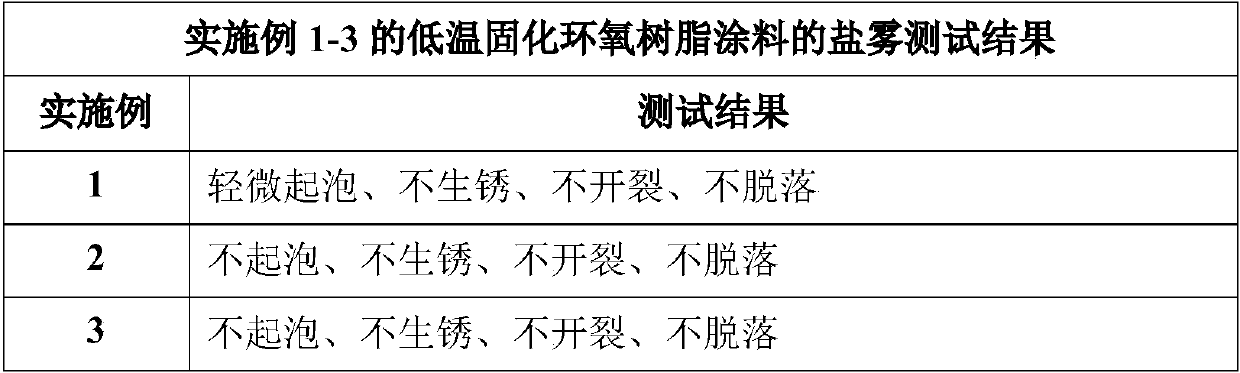
InactiveCN109749581AIncrease the reaction speed at low temperaturePerformance unchangedEpoxy resin coatingsEpoxyLow temperature curing
The invention discloses low-temperature cured epoxy resin paint which mainly comprises, by weight, 80-120 parts of epoxy resin, 5-30 parts of di-n-butylamine, 30-70 parts of n-butyl acetate and 30-70parts of hydroxy-NCO curing agents. A preparation method of the paint includes the steps:(1) n-butyl acetate heating:heating the n-butyl acetate to reach the temperature of 75-90 DEG C; (2) epoxy resin dis: adding the epoxy resin into the n-butyl acetate and stirring and dissolving the epoxy resin to obtain epoxy resin (3) di-n-butylamine adding: dropping the di-n-butylamine into the epoxy resin solution at the temperature of 75-85 DEG C;(4) heat preservation: preserving heat for 1.5-3 hours after dropping to obtain a finished product. As the hydroxy-NCO curing agents are adopted and an epoxygroup on the epoxy resin is changed into hydroxy through the di-n-butylamine, an epoxy-hydroxy-NCO curing system is formed in curing under the action of the hydroxy-NCO curing agents, low-temperaturereaction speed is greatly increased, and performances are basically kept unchanged.
Owner:广东科鼎功能材料有限公司
Method for synthesizing heteroatom substituted MeAPO-31 molecular sieve by microwave heating
InactiveCN102001682BShorten crystallization timeLow costMolecular-sieve and base-exchange phosphatesMolecular-sieve silicoaluminophosphatesPhosphoric acidPetrochemical
The invention discloses a method for synthesizing a heteroatom substituted MeAPO-31 molecular sieve by microwave heating, relating to a preparation method of a MeAPO-31 molecular sieve. The invention aims to solve the technical problems that the traditional method for preparing the MeAPO-31 molecular sieve has long crystallization time, easy generation of mixed crystals in products, limited type and quantity of the heteroatom introduced into a frameworkd, the narrow adjustable range of the produced acid sites and the like. The method comprises the following steps of: firstly, preparing gel byusing phosphoric acid, water, di-n-butylamine, aluminum isopropoxide (or boehmite) and metal salt; heating with microwaves; and crystallizing, then centrifugally filtering, washing, drying and roasting to obtain the MeAPO-31 molecular sieve. Compared with the traditional hydrothermal process, the method disclosed in the invention obviously shortens the crystallization time of the molecular sieve and does not generate mixed crystals. The MeAPO-31 molecular sieve prepared with the method can be used as catalysts in the fields of petrochemical industry, fine chemical industry and the like.
Owner:HEILONGJIANG UNIV
Polyurethane acrylate curable paint synthesizing method
InactiveCN104530957AImprove adhesionImprove low temperature resistancePolyurea/polyurethane coatingsHydroxybenzoate Ethers(Hydroxyethyl)methacrylate
The invention relates to a synthesizing method of a polyurethane acrylate curable paint. The method comprises the following steps: polyoxypropylenetriol is subjected to a reduced-pressure vacuum treatment; pretreated toluene diisocyanate and 1,6-hexanediol diacrylate are added into a three-necked flask according to a formula, and are well mixed by stirring; polyoxypropylenetriol is added into the three-necked flask in batches and is subjected to a reaction; isocyano content is determined with a di-n-butylamine method after the reaction; hydroxyethyl methacrylate contaning p-hydroxyanisole and a catalyst is added into the three-necked flask and is subjected to a reaction; the product is cooled to room temperature and is discharged, such that a polyurethane acrylate oligomer is obtained; the polyurethane acrylate oligomer, an active diluting monomer, and auxiliary agents such as a coupling agent with amounts according to the formula are weighed and sequentially added into a flask; the mixture is well dispersed by stirring; a photoinitiator is added under a situation protected from light, and the mixture is well mixed, such that the polyurethane acrylate curable paint is obtained. The method provided by the invention is simple, and has low cost. The synthesized polyurethane acrylate curable paint has good adhesion, low-temperature resistance, and excellent wear resistance.
Owner:CHONGQING XUXING CHEM
High-quality organic glass
The invention disclose high-quality organic glass which comprises organic glass, a polymerization stabilizer, a dispersant, a molecular weight regulator, a flexibilizer, an abrasion-resistant agent and a heat-proof agent, wherein the polymerization stabilizer is sodium dimethyl dithio carbamate, the dispersant is lauryl sodium sulfate, the molecular weight regulator is di-n-butylamine, the flexibilizer is polyvinyl acetate, the abrasion-resistant agent is epoxy resin, and the heat-proof agent is ethylene propylene diene monomer. According to the high-quality organic glass, the polymerization stabilizer, the dispersant, the molecular weight regulator, the flexibilizer, the abrasion-resistant agent and the heat-proof agent are added into the organic glass, so that the high-quality organic glass has the characteristics of being high in production efficiency, excellent in product quality, high in tenacity, resistant to scraping abrasion, good in heat resistance, and long in service life.
Owner:ZHANGJIAGANG DELITE NEW MATERIAL
Morpholine-substituted poly(arylene ether) and method for the preparation thereof
InactiveUS8017716B2Improve compatibilityStrong bias toward incorporationAluminium compoundsPlastic/resin/waxes insulatorsPolymer scienceMorpholine
A poly(2,6-dimethyl-1,4-phenylene ether) prepared using a morpholine-containing polymerization catalyst has a monomodal molecular weight distribution with a reduced content of very high molecular weight species. It also exhibits increased morpholine incorporation in the high molecular weight fraction. Compared to commercially available poly(2,6-dimethyl-1,4-phenylene ether) prepared using a di-n-butylamine-containing polymerization catalyst, the poly(2,6-dimethyl-1,4-phenylene ether) of the invention exhibits reduced odor. Compared to other poly(2,6-dimethyl-1,4-phenylene ether) prepared using a morpholine-containing polymerization catalyst, the poly(2,6-dimethyl-1,4-phenylene ether) of the invention exhibits improved molecular weight build during compounding and improved compatibilization with polyamides.
Owner:SABIC GLOBAL TECH BV
Tanning agent for cow skins and cow skin tanning process
InactiveCN107419040AWith cellulite effectIncrease elasticityLeather manufacturingTanning treatmentFat burningCis-Butenedioic Acid
The invention relates to a tanning agent for cow skins and a cow skin tanning process. The tanning agent consists of methacrylic acid, resorcinol, urea resins, 2-oxopyrrolidin, dibutyl itaconate, bicarboxyethyl amine, carboxybenzaldehyde, di-n-butylamine, fatty aldehyde and maleic acid. The tanning process can perform the procedures of water leaching, fleshing, softening, tanning and airing on the cow skins. The tanning agent achieves an excellent tanning effect on the cow skins in a targeted manner; the tanning agent is adopted to tan the cow skins to effectively simplify the procedures; cow leathers can be obtained by only performing the procedures of water leaching, fleshing, softening, tanning and airing on the cow skins; the tanning procedure achieves a fat burning effect on the cow skins; the tanning process is effectively simplified; the tanning efficiency is effectively improved; the production cost is effectively reduced; and the produced cow leathers have such excellent characteristics as softness, fineness, wear resistance and high elasticity.
Owner:QUANZHOU XINCAI TRADE
Preparation method of N,N-di-n-butylethylenediamine
InactiveCN103012157AHarm reductionHigh yieldAmino preparation by functional substitutionSodium methoxideReaction temperature
The invention discloses a preparation method of N,N-di-n-butylethylenediamine, which comprises the following steps: 1) adding a sodium methoxide methanol solution to carry out reaction on raw materials di-n-butylamine and 2-chlorethamin hydrochloride in a high-pressure autoclave, wherein the mol ratio of di-n-butylamine to 2-chlorethamin hydrochloride is (4-6):1, the mol ratio of sodium methoxide to 2-chlorethamin hydrochloride is (0.5-2):1, the reaction temperature is 100-200 DEG C, the reaction time is 2-9 hours, and the reaction pressure is 0.5-1.0 MPa; and 2) adding alkali solution into the obtained reaction solution in the step 1) to regulate the pH value to 12-13, and carrying out liquid separation and rectification to obtain the N,N-di-n-butylethylenediamine. The method disclosed by the invention has the characteristics of simple technique, high yield and low cost.
Owner:ZHEJIANG UNIV
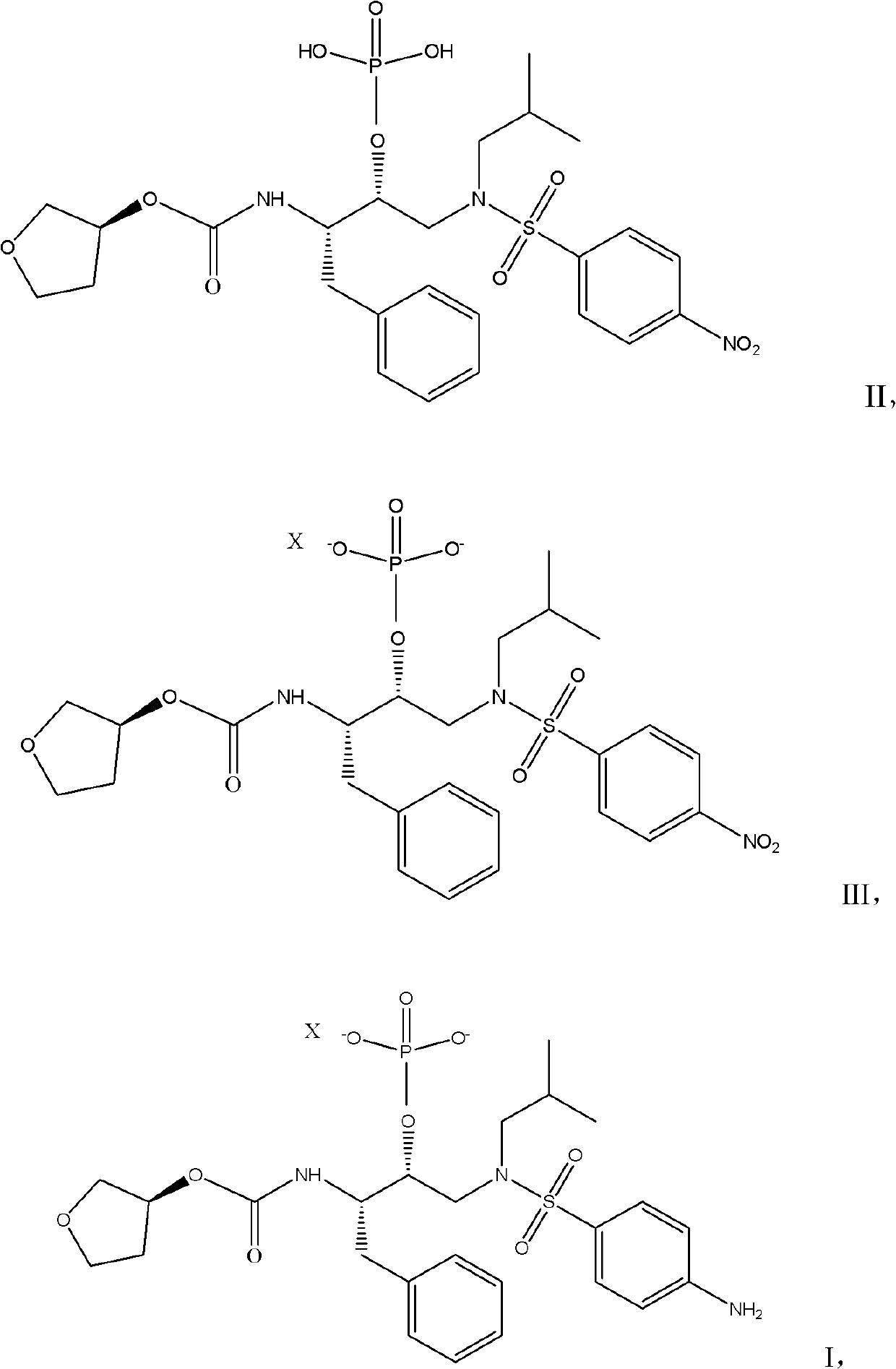
Preparation method of fosamprenavir derivative and related intermediate thereof
ActiveCN102453054ALow costHigh yieldGroup 5/15 element organic compoundsPalladium on carbonN-Butanol
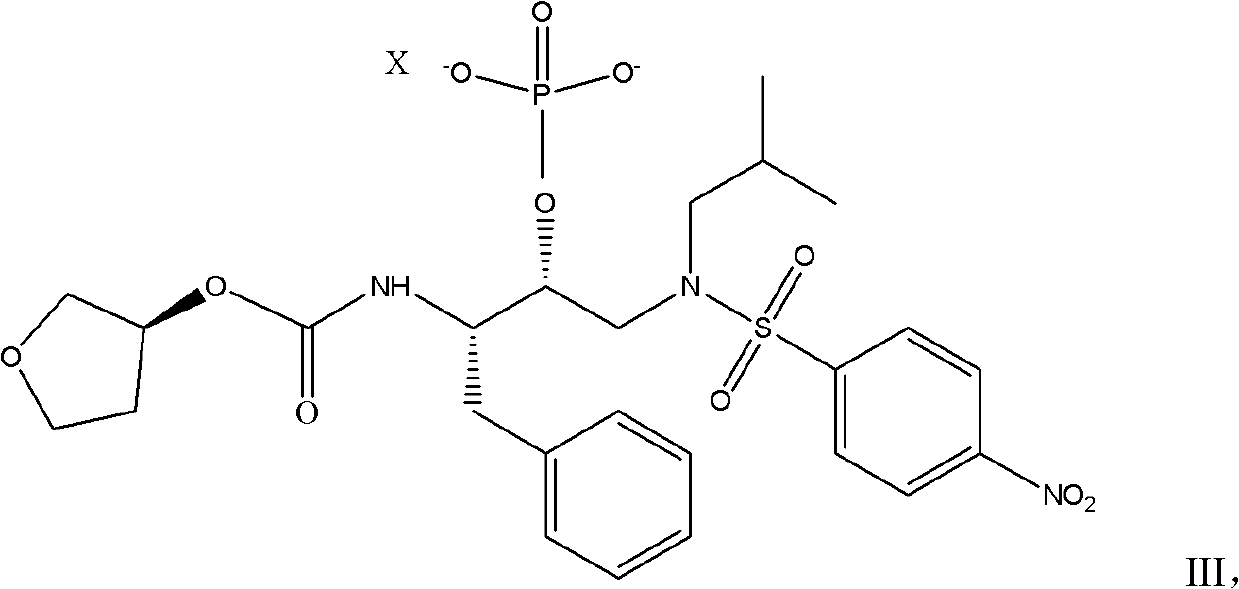
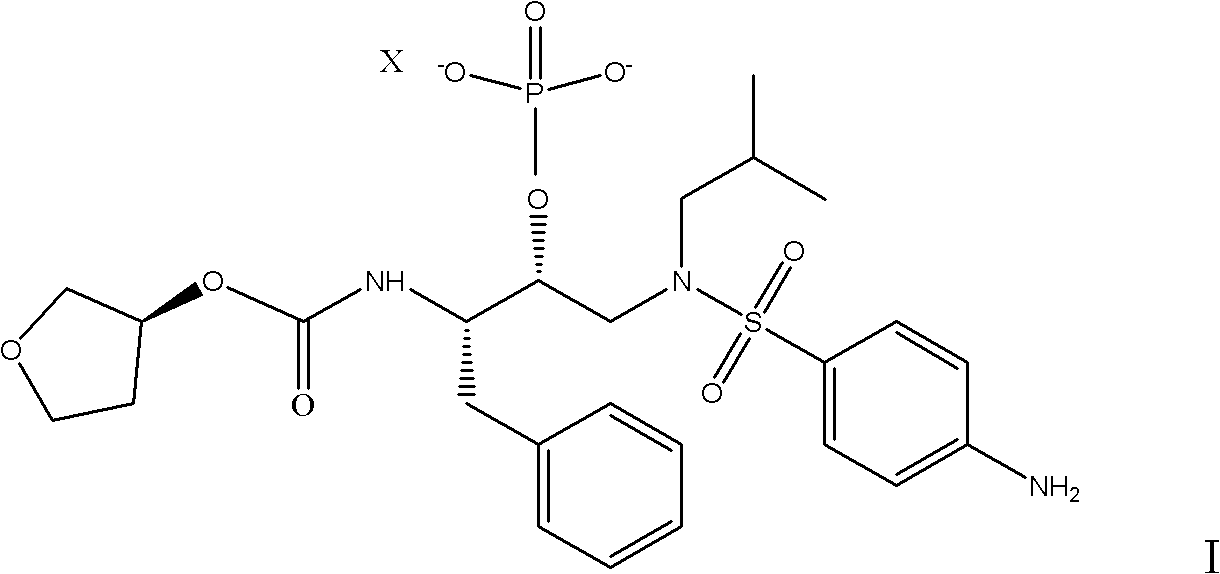
The invention relates to s preparation method of a fosamprenavir derivative, which comprises the following steps: reacting a compound disclosed as Formula II or ammonium salt thereof with a metallic ion source in a solvent to obtain a compound disclosed as Formula III, separating or purifying, and carrying out catalytic reduction on the compound disclosed as Formula III to obtain a compound disclosed as Formula I, wherein X is a metallic ion, the metallic ion source is preferably a calcium ion source, sodium ion source or potassium ion source, and X is preferably a calcium ion, sodium ion or potassium ion; the solvent is methanol, ethanol, n-propanol, isopropanol, n-butanol or sec-butanol; the catalytic reduction is carried out by using palladium-on-carbon as a catalyst and hydrogen as a reducer; and the ammonium salt is a monomethyl amine salt, dimethyl amine salt, monoethyl amine salt, diethylamine salt, isopropylamine salt, di-n-butylamine salt, dipropyl amine salt, tert-butylamine salt or dicyclohexyl amine salt. The invention also provides a related intermediate of the preparation method of the fosamprenavir derivative. The preparation method of the fosamprenavir derivative saves the cost, enhances the yield, and is suitable for large-scale popularization.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com