Preparation method of fosamprenavir derivative and related intermediate thereof
A technology of fosamprenavir and derivatives, which is applied in the field of preparation of fosamprenavir derivatives and fosamprenavir derivatives, can solve the problems of high cost and low yield, and achieves improved yield, cost saving, Suitable for large-scale promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
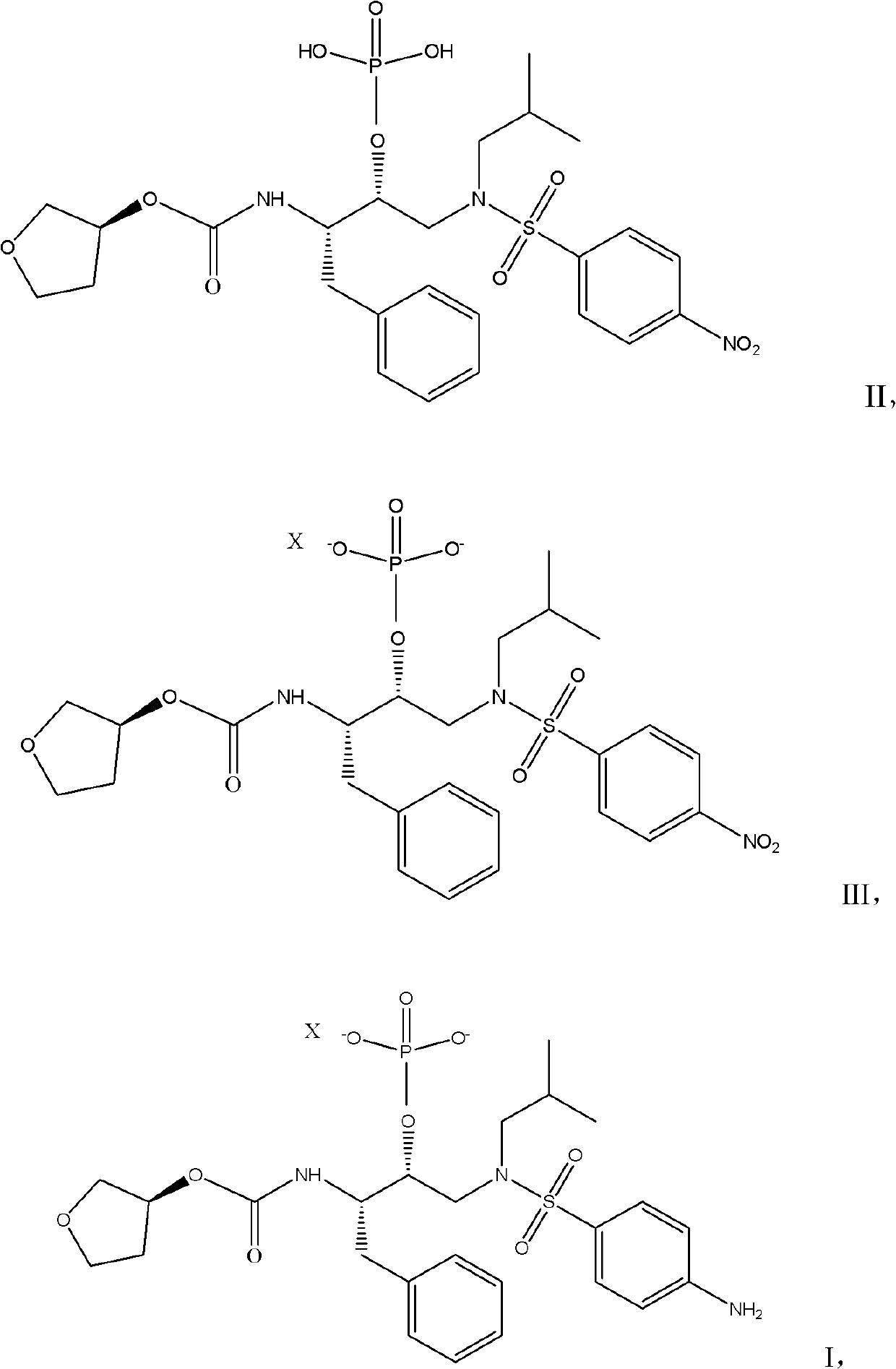
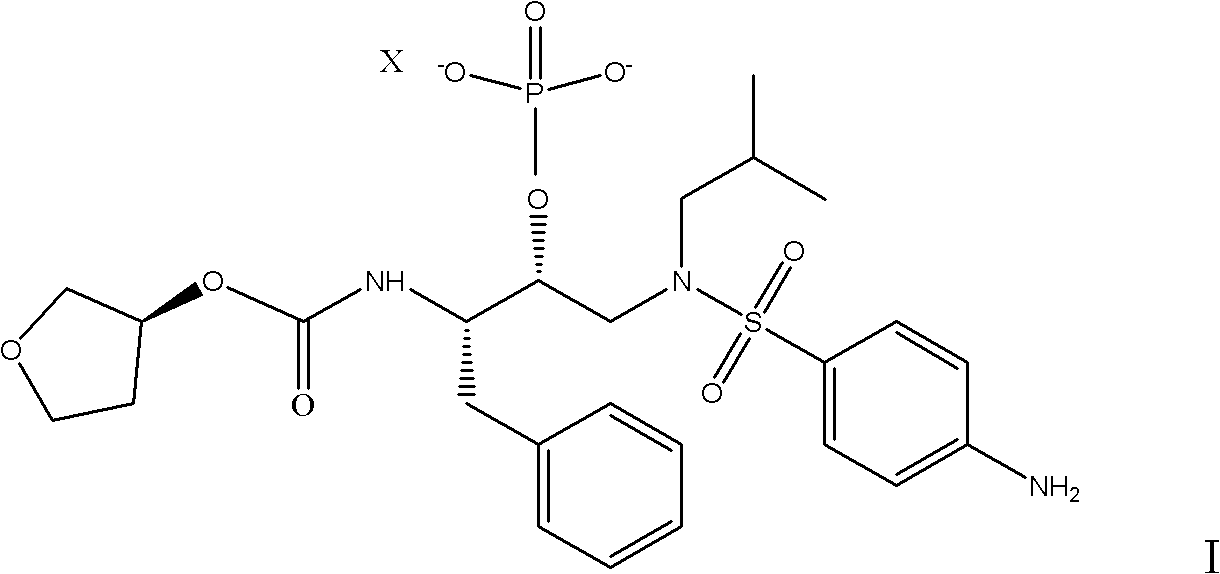
[0025] 15g (3S) Tetrahydro-3-furyl (1S, 2R)-3-[[(4-aminophenyl)-sulfonyl](isobutyl)amino-1-benzyl-2-(phosphono Oxy)propyl-carbamate was dissolved in 45ml of methanol and heated to 55°C, and a mixture of 4.2g of calcium acetate monohydrate and 150ml of water was added dropwise. After the addition was completed, it was kept at 55°C for half an hour. Cool to 20°C, filter and dry.
[0026] The dry product was dissolved in 200 ml of methanol, 0.3 g of 10% Pd / C was added, and the mixture was stirred under hydrogen for 20 hours. The catalyst was filtered off.
[0027] After the filtrate was concentrated under reduced pressure to the remaining 100ml, it was dropped into 150ml of water at room temperature to crystallize. After the drop was completed, it was stirred at room temperature for half an hour, filtered, and dried in vacuo to obtain 13.2g of calcium (3S) tetrahydro-3-furyl (1S, 2R)-3 -[[(4-Aminophenyl)-sulfonyl](isobutyl)amino-1-benzyl-2-(phosphonooxy)propyl-carbamate. Yield...
Embodiment 2
[0029] In a clean and dry 250ml four-necked flask, put 10g (3S) tetrahydro-3-furyl (1S, 2R)-3-[[(4-aminophenyl)-sulfonyl](isobutyl)amino- 1-Benzyl-2-(phosphonooxy)propyl-carbamate, add 30ml of absolute ethanol to dissolve, and heat to 50°C, add dropwise a mixture of 2.8g sodium acetate and 100ml water, drop After the addition, keep the temperature at 50°C for half an hour. After the heat preservation is completed, evaporate the solvent to dryness under reduced pressure in a water bath at 60°C, add 100ml of methyl tert-butyl ether for beating and crystallization, filter and dry.
[0030] In a clean and dry autoclave, put the dried material, 120ml methanol and 0.2g10% Pd / C, close the autoclave, first replace with nitrogen three times, then replace with hydrogen three times, increase the hydrogen pressure to 1.0Mpa, and pass hydrogen at room temperature 24 hours, until the reaction of compound 2 is complete, vent, take out the material, filter, and concentrate the filtrate to dry...
Embodiment 3
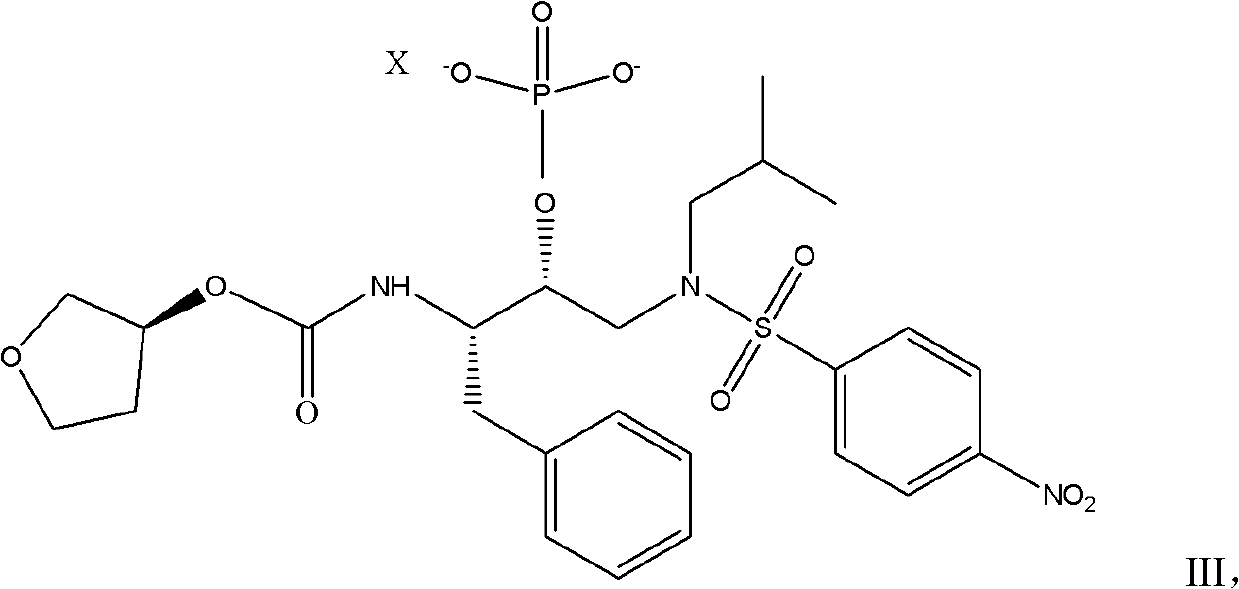
[0032] 10.5g (3S) Tetrahydro-3-furyl (1S, 2R)-3-[[(4-nitrophenyl)-sulfonyl] (isobutyl) amino-1-benzyl-2-( Diethylamine salt (II) of phosphonooxy)propyl-carbamate, dissolved in 30ml of absolute ethanol, and heated to 50°C, added dropwise a mixture of 2.8g of calcium acetate monohydrate and 100ml of water , after the dropwise addition, keep warm at 50°C for half an hour, then cool to room temperature, filter and dry.
[0033] Dissolve the dry product in 120ml of propanol and 0.2g of 10% Pd / C, close the autoclave, first replace it with nitrogen three times, then replace it with hydrogen three times, raise the pressure of hydrogen to 1.0Mpa, and pass hydrogen at room temperature for 24 hours until the reaction is complete, then vent it, The material was taken out, filtered, and after the filtrate was concentrated under reduced pressure to the remaining 60ml, it was dropped into 100ml of water for crystallization at room temperature. , 2R)-3-[[(4-aminophenyl)-sulfonyl](isobutyl)am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com