Method for determining content of mono-ester aconitum alkaloids in ephedra-aconitum carmichaelii-liquorice preparation
A monoester-type alkaloid and determination method technology, which is applied in the field of pharmaceuticals, can solve the problems of low precision and achieve the effects of good durability and improved tailing factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Determination of Monoester Alkaloid Content in Mafugan Granules
[0101] 1) Preparation of granules:
[0102] Take 6 kg of aconite, 2 kg of ephedra, and 2 kg of licorice, add water to decoct twice, add 8 times the amount of water for the first time, decoct for 2 hours, add 6 times the amount of water for the second time, decoct for 1 hour, combine the two decoctions, Filter, concentrate to a thick paste, dry under reduced pressure below 80°C, pulverize, mix with appropriate amount of dextrin, make granules, dry, and granulate to obtain the granules of Mafugan drug.
[0103] 2) Content determination:
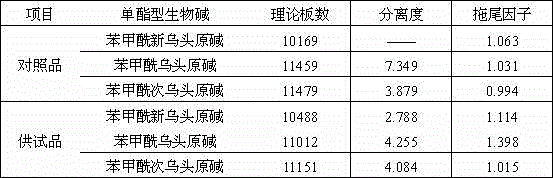
[0104] Chromatographic conditions and system suitability test: the chromatographic column is filled with polar ether-linked phenyl-bonded silica gel (Welch Ultimate Phenyl-Ether, 250×4.6mm, 5μm, new column); acetonitrile: 0.05% phosphoric acid solution (18: 82) is the mobile phase, add 0.2ml of triethylamine and 0.1ml of di-n-butylamine for every 1000ml of 0.0...
Embodiment 2
[0111] Embodiment 2: Determination of content of monoester type alkaloids in Mafugan Granules
[0112] 1) Preparation of granules:
[0113] Take 12kg aconite, 10kg ephedra, and 10kg licorice, add water to decoct twice, add 10 times the amount of water for the first time, decoct for 2.5 hours, add 8 times the amount of water for the second time, decoct for 1.5 hours, combine the two decoctions, Filter, concentrate to a thick paste, dry under reduced pressure below 80°C, pulverize, mix with appropriate amount of dextrin, make granules, dry, and granulate to obtain the granules of Mafugan drug.
[0114] 2) Content determination:
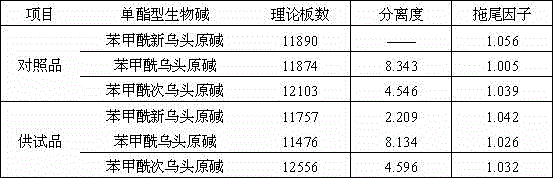
[0115] Chromatographic conditions and system suitability test: the chromatographic column is filled with polar ether-linked phenyl-bonded silica gel (Welch Ultimate Phenyl-Ether, 30×2.1mm, 3μm, used for 1 month); acetonitrile: 0.2% phosphoric acid solution ( 25:75) is the mobile phase, in which the phosphoric acid solution contains 0.06% triethylamine...
Embodiment 3
[0122] Embodiment 3: Determination of content of monoester type alkaloids in Mafugan Capsules
[0123] 1) Preparation of capsules:
[0124] Take 10kg aconite, 6.67kg ephedra, and 6.67kg licorice, add water to decoct twice, add 8 times the amount for the first time, decoct for 3 hours, add 6 times the amount for the second time, decoct for 2 hours, filter the decoction, and the filtrate Combined, concentrated to a thick paste, dried under reduced pressure below 80°C, pulverized, mixed with an appropriate amount of starch, packed into capsules to obtain Mafugan capsules.
[0125] 2) Content determination:
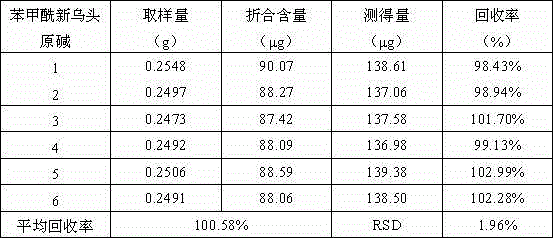
[0126] Chromatographic conditions and system suitability test: the chromatographic column is filled with polar ether-linked phenyl-bonded silica gel (Agela Venusil XBP Polar-Phenyl, 100×4.6mm, 5μm, new column); acetonitrile: 0.1% phosphoric acid solution (20 : 80) is the mobile phase, in which the phosphoric acid solution contains 0.03% triethylamine and 0.02% di-n-butylami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com