Preparation process for tetrabutyl urea
A technology of tetrabutylurea and preparation process is applied in the preparation of urea derivatives, the preparation of organic compounds, organic chemistry, etc., and can solve the problems of only 80%-85% yield of finished products, many by-products, and difficult industrialization. , to achieve the effect of cheap raw materials, good product quality and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1), add di-n-butylamine, phosgene and 20% sodium hydroxide solution in a molar ratio of 2: 1: 2 in a reaction vessel;
[0019] (2), the acylation reaction temperature is 60°C, and the acylation reaction time is 5 hours, and the crude product of tetrabutylurea is obtained;
[0020] (3) After the crude tetrabutylurea was left to stand for 30 minutes, the mother liquid water in the lower layer was separated, and the crude tetrabutylurea was distilled at a vacuum degree of -0.092MPa and a kettle temperature of 200°C to obtain the final product of tetrabutylurea .
Embodiment 2
[0022] (1), add di-n-butylamine, phosgene and 20% sodium hydroxide solution in a molar ratio of 2: 1.05: 2.1 in the reaction vessel;
[0023] (2), the acylation reaction temperature is 70°C, and the acylation reaction time is 6 hours, and the crude product of tetrabutylurea is obtained;
[0024] (3) After the crude tetrabutylurea was left to stand for 30 minutes, the mother liquid water in the lower layer was separated, and the separated crude tetrabutylurea was distilled at a vacuum degree of -0.094MPa and a kettle temperature of 210°C to obtain the final product of tetrabutylurea .
Embodiment 3
[0026] (1), add di-n-butylamine, phosgene and 20% sodium hydroxide solution in a molar ratio of 2: 1.05: 2.1 in the reaction vessel;
[0027] (2), the acylation reaction temperature is 80°C, and the acylation reaction time is 8 hours, and the crude product of tetrabutylurea is obtained;
[0028] (3) After the crude tetrabutylurea was left to stand for 30 minutes, the mother liquid water in the lower layer was separated, and the separated crude tetrabutylurea was distilled at a vacuum degree of -0.096MPa and a kettle temperature of 220°C to obtain the final product of tetrabutylurea .
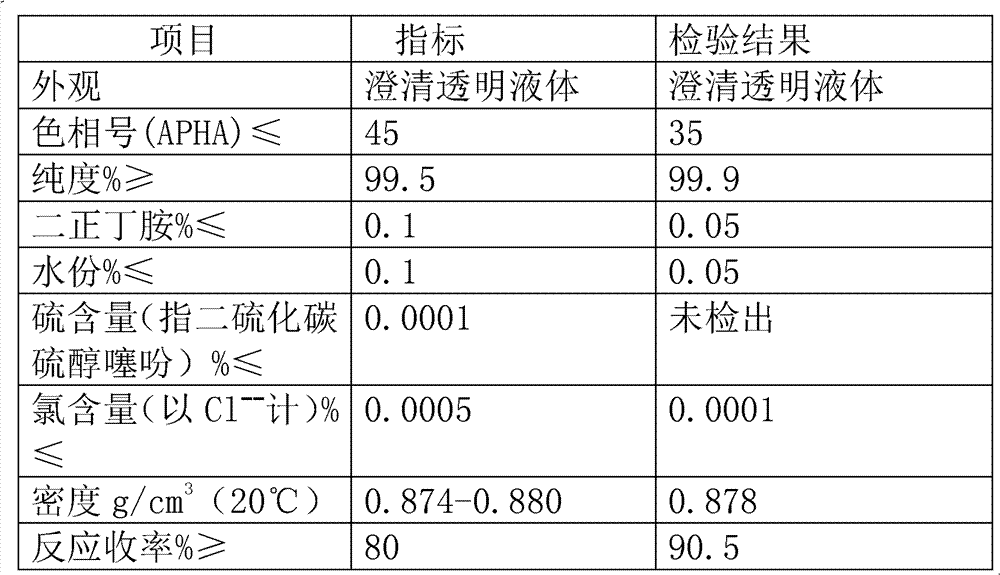
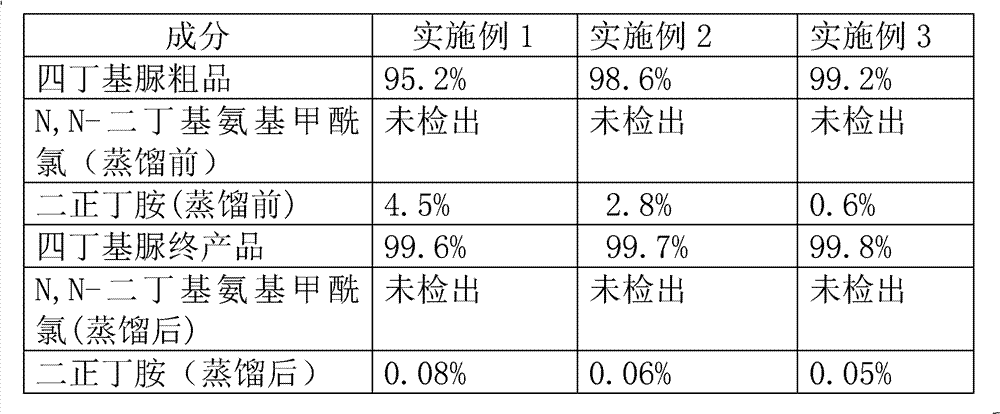
[0029] The detection results of three embodiments are as follows:
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com