Preparation method for sulfur-containing carbofuran derivative with less than 0.1% of harmful impurity carbofuran
A technology of derivatives and carbosulfur, applied in the field of preparation of sulfur-containing carbosulfur derivatives, can solve problems such as hindering the popularization and application of carbosulfur-containing sulfur derivatives, and achieve the effect of broad prospects for popularization and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
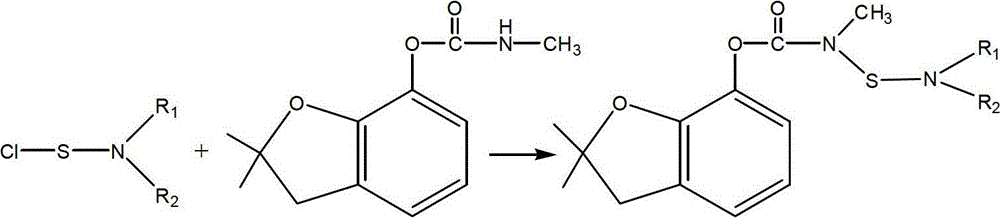
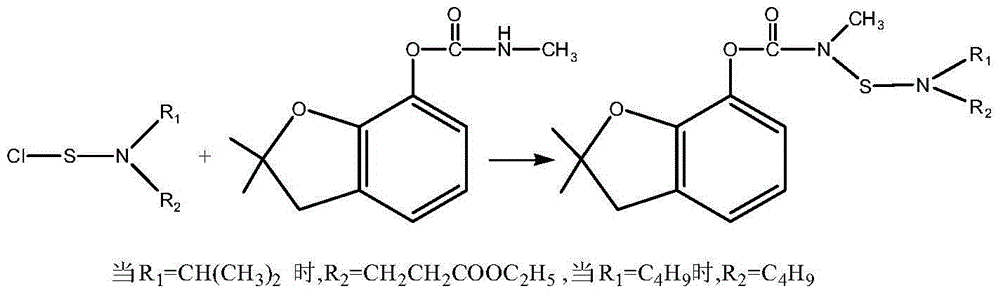
[0013] Carbofuran, chemical name N-[2,3-dihydro-2,2-dimethylbenzofuran-7-yloxycarbonyl(methyl)aminothio]-N-isopropyl-β - Preparation of ethyl alanine
[0014] Add 1L 1,2-dichloroethane, 159g (1mol, 166mL) N-isopropyl-β-alanine ethyl ester and 101g (1mol) triethylamine into a stirred reactor, cool to 0 ℃. Add 67.5g (0.5mol) of sulfur monochloride dropwise under stirring, and react at 0-10°C for 1 hour after dropping. After the reaction, wash with water, separate the 1,2-dichloroethane layer, and concentrate under reduced pressure until 0.8 L of 1,2-dichloroethane is removed. Then lower the temperature to 5°C again, add 67.5g (0.5mol) sulfuryl chloride dropwise under stirring, react at 0-30°C for 3 hours after the dripping, add 210g (0.95mol) carbofuran, 20g (0.2mol) ) N-methylpyrrolidone and 0.3L 1,2-dichloroethane, cooled to -10°C, and 202g (2mol) triethylamine was added dropwise under stirring, and stirred at -10°C for 5 hours after the dropping, after the reaction was com...
Embodiment 2
[0016] Carbofuran, chemical name N-[2,3-dihydro-2,2-dimethylbenzofuran-7-yloxycarbonyl(methyl)aminothio]-N-isopropyl-β - Preparation of ethyl alanine
[0017] Add 1.5L 1,2-dichloroethane, 159g (1mol, 166mL) N-isopropyl-β-alanine ethyl ester and 101g (1mol) triethylamine into a stirred reactor, cool to 0°C. Add 67.5g (0.5mol) of sulfur monochloride dropwise under stirring, and react at 0-10°C for 1 hour after dropping. After the reaction, wash with water, separate the 1,2-dichloroethane layer, and concentrate under reduced pressure until 0.8 L of 1,2-dichloroethane is removed. Then lower the temperature to 5°C again, add 67.5g (0.5mol) of sulfuryl chloride dropwise under stirring, react at 0-30°C for 3 hours after the dropping, add 210g (0.95mol) of carbofuran, 51.6g (0.5mol) mol) N-methylpyrrolidone and 0.3L 1,2-dichloroethane, cool to 0°C, add 202g (2mol) triethylamine dropwise under stirring, and stir at 0~10°C for 3 hours after dropping, the reaction is complete After w...
Embodiment 3
[0019] Carbofuran, chemical name N-[2,3-dihydro-2,2-dimethylbenzofuran-7-yloxycarbonyl(methyl)aminothio]-N-isopropyl-β - Preparation of ethyl alanine
[0020] Add 1.3L 1,2-dichloroethane, 159g (1mol, 166mL) N-isopropyl-β-alanine ethyl ester and 101g (1mol) triethylamine into a stirred reactor, cool to 0°C. Add 67.5g (0.5mol) of sulfur monochloride dropwise under stirring, and react at 0-10°C for 1 hour after dropping. After the reaction, wash with water, separate the 1,2-dichloroethane layer, and concentrate under reduced pressure until 0.8 L of 1,2-dichloroethane is removed. Then lower the temperature to 5°C again, add 67.5g (0.5mol) sulfuryl chloride dropwise under stirring, and react at 0-30°C for 3 hours after the dropwise completion, add 210g (0.95mol) carbofuran, 47.4g (0.6 mol) dimethylformamide and 0.3L 1,2-dichloroethane, cooled to 0°C, and 202g (2mol) triethylamine was added dropwise under stirring, and stirred at 30°C for 2 hours after the dripping, after the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com