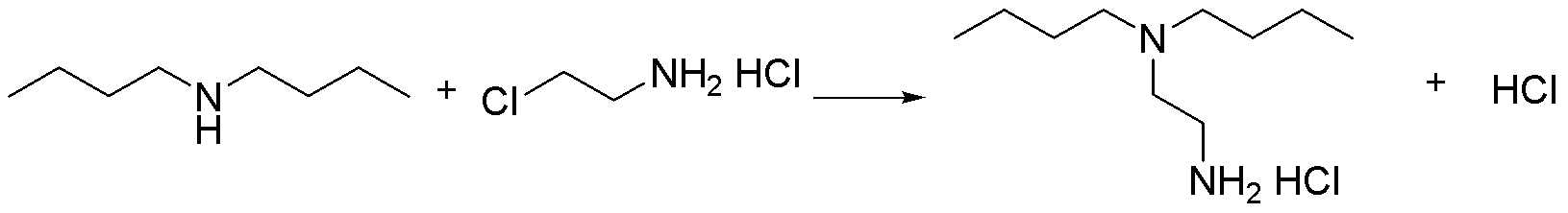Preparation method of N,N-di-n-butylethylenediamine
A technology of di-n-butylethylenediamine and di-n-butylamine, which is applied in the field of synthesis of organic compounds, can solve the problems of long reaction time, cumbersome process, and inconvenient operation, and achieves low environmental hazards, simple process flow, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, a preparation method of N,N-di-n-butylethylenediamine, using di-n-butylamine and 2-chloroethylamine hydrochloride as starting materials, the following steps are carried out in sequence:
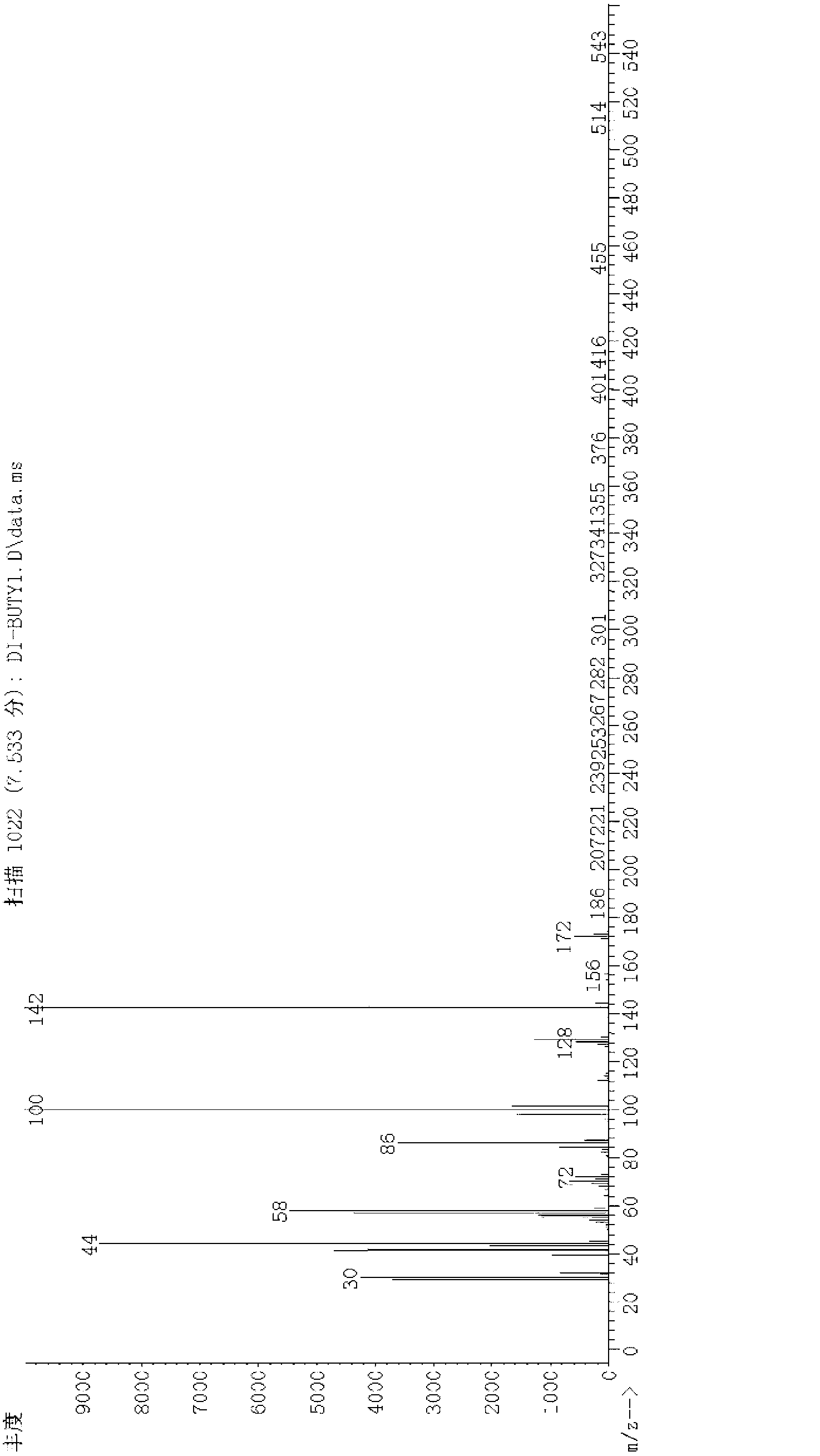
[0018] In autoclave, add di-n-butylamine 85ml (0.5mol), 2-chloroethylamine hydrochloride 11.6g (0.1mol), the methanol solution of the sodium methylate of mass concentration 30% (which contains sodium methylate 5.4g (0.1 mol), methanol 16ml), close the kettle lid. The temperature was raised to 150° C., and the reaction pressure was 0.8 MPa at this time, and the reaction was maintained at this temperature for 5 hours. After the reaction, add saturated aqueous sodium hydroxide solution to the reaction solution to adjust the pH to 12, take the upper oil phase for rectification, collect fractions at 109-114°C, and obtain 77.9 g of the product N,N-di-n-butylethylenediamine, The yield was 74.7%, and the structure of the obtained product was confirmed by mass spectrometry.
Embodiment 2~6
[0020] Change the following reaction conditions in embodiment 1: di-n-butylamine consumption (abbreviation V 1 ), the amount of sodium methoxide (M 2 ), methanol dosage (V 2 ), reaction temperature (abbreviated T), reaction time (abbreviated t), reaction pressure (abbreviated P); 1 ), to obtain Examples 2-6, thereby obtaining the corresponding yield of N,N-di-n-butylethylenediamine (abbreviated as y). See Table 1 for details and data results.
[0021] Table 1
[0022] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com