Organic molecular ferroelectric crystal di-n-butylamine difluoromonochloroacetate, preparation method therefor and use of organic molecular ferroelectric crystal di-n-butylamine difluoromonochloroacetate
A technology of di-n-butylamine difluoro-monochloroacetate and n-butylamine difluoro-monochloroacetate is applied in organic molecular ferroelectric crystal di-n-butylamine difluoro-monochloroacetate, the ferroelectric crystal In the field of material preparation, it can solve the problems of small spontaneous polarization, practical application limitations, and poor material stability, and achieve the effects of low cost, simple reaction and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0024] At room temperature, dissolve di-n-butylamine in deionized water, then add difluoro-chloroacetic acid, stir fully at 40-60°C (preferably 50°C) until it is completely dissolved, filter, and place the filtrate in a 35-45 °C (preferably 40 °C), slow crystallization in an oven to obtain the di-n-butylamine difluorochloroacetic acid salt, and the molar ratio of di-n-butylamine to difluorochloroacetic acid is 1:1. The molar ratio of di-n-butylamine to difluoro-chloroacetic acid can also be around 1:1.
[0025] (2) Specific examples
Embodiment 1
[0027] Preparation of organic molecular ferroelectric crystal material di-n-butylamine difluoromonochloroacetate
[0028] At room temperature, di-n-butylamine (5×10 -3 mol) and difluorochloroacetic acid (5×10 -3 mol) was dissolved in 50 mL of distilled water, heated to 50°C, and stirred for 20 min to fully react. After the solution is cooled and crystallized, filtered and dried, the di-n-butylamine difluoromonochloroacetate is obtained. Then the solid powder was redissolved in 50 mL of distilled water, recrystallized three times, and slowly volatilized to obtain the colorless and transparent strip-shaped target product, namely di-n-butylamine difluoro-chloroacetate compound, with a yield of 96.2%.
[0029] After testing, the chemical formula of the di-n-butylamine difluoromonochloroacetate is C 10 h 20 CIF 2 NO 2 , its structure is
[0030]
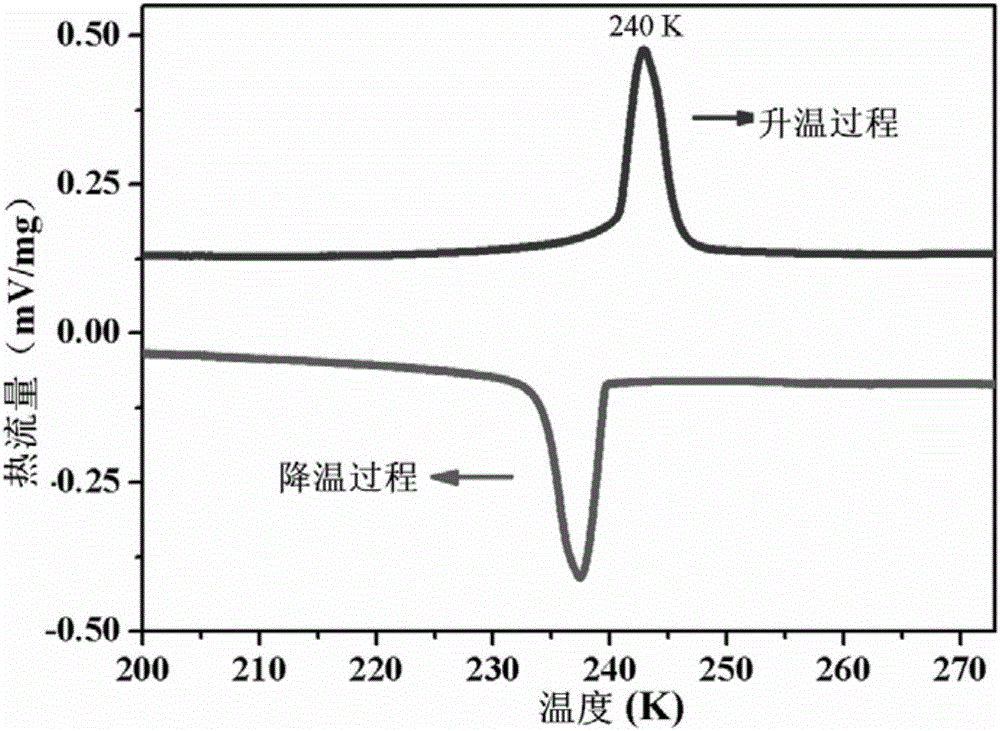
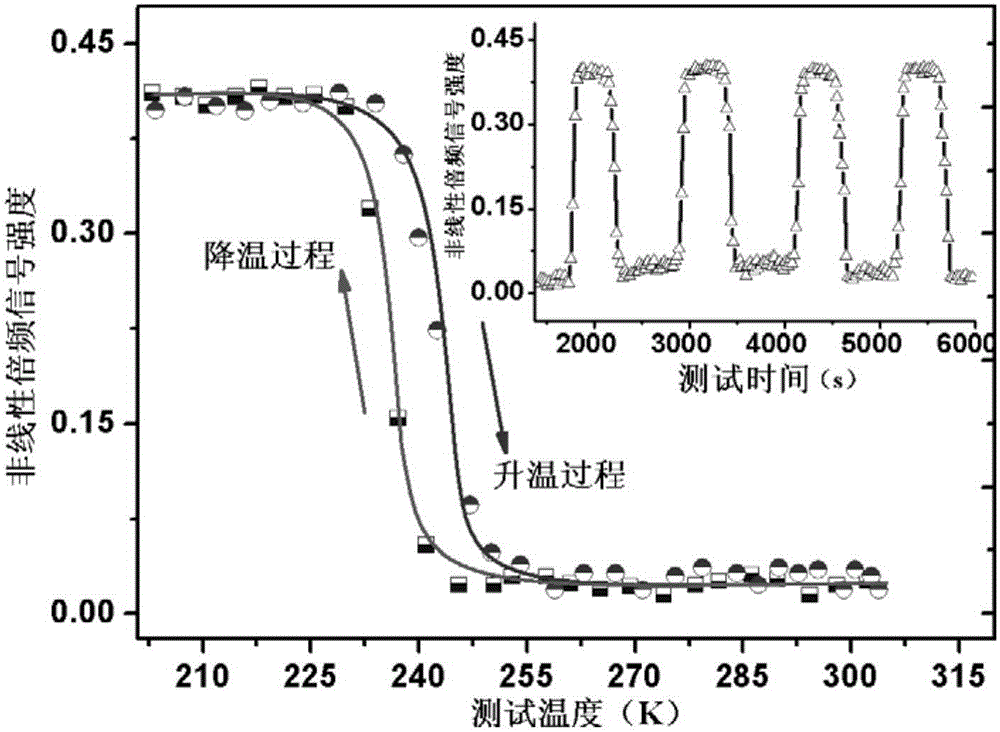
[0031] The di-n-butylamine difluoro-chloroacetate belongs to the orthorhombic crystal system at room temperature, and the spac...
Embodiment 2
[0034] Application of organic molecular ferroelectric crystal material di-n-butylamine difluoromonochloroacetate in the field of ferroelectric storage
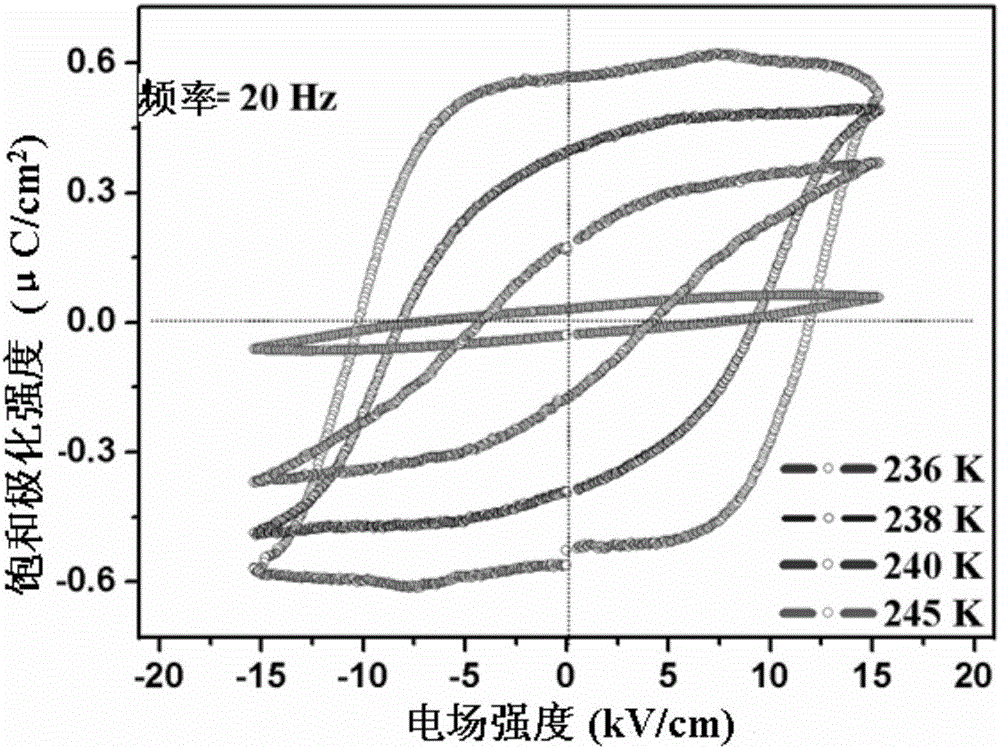
[0035] The di-n-butylamine difluoro-chloroacetate obtained in embodiment 1 is carried out hysteresis loop test, such as figure 1 As shown, at 245K, the test frequency is 20Hz, and the polarization intensity of the material has a linear relationship with the electric field; during the cooling process, under the same test frequency, there is an obvious hysteresis loop characteristic between the polarization intensity and the electric field at 240K ;The temperature continues to decrease, the polarization intensity continues to increase, and the coercive field also increases continuously, and the saturation polarization intensity reaches the saturation value of 3.9μC / cm at 236K 2 , the coercive field is 12.4kV cm -1 . The large saturation polarization and moderate coercive field make this organic molecular ferroelectric crystal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com