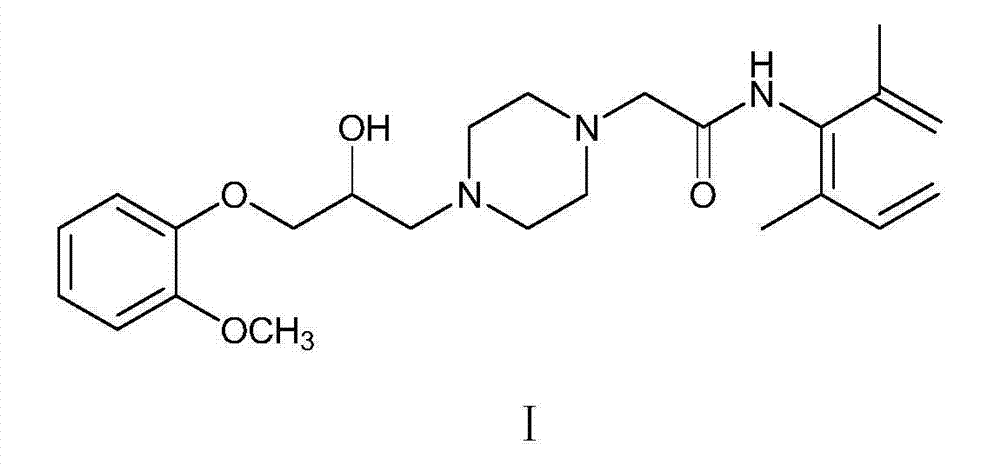
Ranolazine sustained release tablet medicine composition and preparation method thereof
The technology of a composition and ranolazine is applied in the pharmaceutical composition of ranolazine sustained-release tablets and its preparation field, which can solve problems such as strong hygroscopicity, poor fluidity, difficult selection of excipients, unstable preparations, etc., so as to facilitate human body absorption and improve The effect of stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
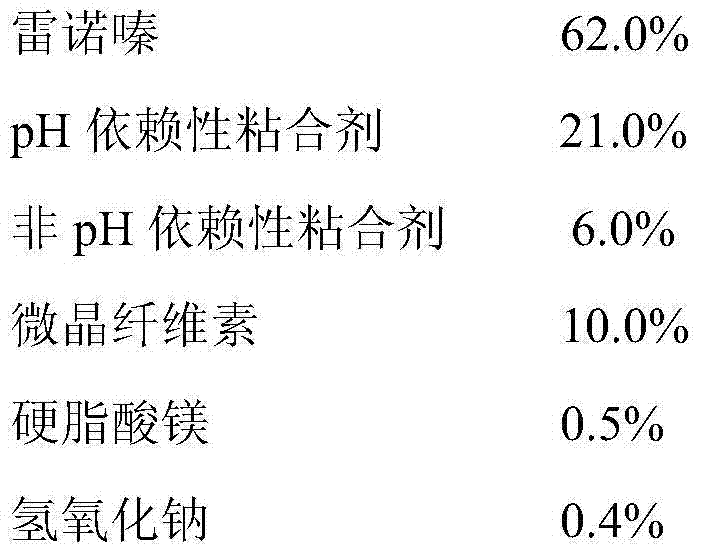
[0047] The preparation of embodiment 1 ranolazine sustained-release tablet pharmaceutical composition
[0048] prescription:
[0049]
[0050] The preparation method is prepared according to the following steps:
[0051] (1) Weigh the ranolazine raw material according to the prescription amount, and pass it through a 60-mesh sieve;
[0052] (2) After mixing the treated ranolazine and microcrystalline cellulose evenly, fluidize in a fluidized bed;
[0053] (3) Take the prescription amount of NaOH to prepare an aqueous solution with a mass concentration of 5%, and spray it evenly on the fluidized mixture;
[0054] (4) Prepare hydroxypropyl methylcellulose into an aqueous solution with a concentration of 5% as a binder and spray it in for granulation;
[0055] (5) Take the prescribed amount of Eudragit After diluting L30D-5530% water dispersion with equal weight of water, spray it into the granulated granules to coat the granules;
[0056] (6) Dry the coated granules in ...
Embodiment 2
[0060] The preparation of embodiment 2 ranolazine sustained-release tablet pharmaceutical composition
[0061] prescription:
[0062]
[0063]
[0064] The preparation method is prepared according to the following steps:
[0065] (1) Weigh the ranolazine raw material according to the prescription amount, and pass it through a 60-mesh sieve;
[0066] (2) After mixing the treated ranolazine and microcrystalline cellulose evenly, fluidize in a fluidized bed;
[0067] (3) Take the prescription amount of NaOH to prepare an aqueous solution with a mass concentration of 5%, and spray it evenly on the fluidized mixture;
[0068] (4) Prepare polyvinylpyrrolidone into an aqueous solution with a concentration of 5% as a binder and spray it in for granulation;
[0069] (5) Take the prescribed amount of Eudragit After the L100-55 powder is dissolved in 1.5 times the weight of water, it is sprayed into the granulated granules to coat the granules;
[0070] (6) Dry the coated gra...
Embodiment 3
[0074] The preparation of embodiment 3 ranolazine sustained-release tablet pharmaceutical composition
[0075] prescription:
[0076]
[0077] The preparation method is prepared according to the following steps:
[0078] (1) Weigh the ranolazine raw material according to the prescription amount, and pass it through a 60-mesh sieve;
[0079] (2) After mixing the treated ranolazine and microcrystalline cellulose evenly, fluidize in a fluidized bed;
[0080] (3) Take the prescription amount of NaOH to prepare an aqueous solution with a mass concentration of 5%, and spray it evenly on the fluidized mixture;
[0081] (4) The neutral polyacrylate is prepared into an aqueous solution with a concentration of 5% as a binder and sprayed into it for granulation;
[0082] (5) After taking the prescribed amount of polyvinyl acetate phthalate and diluting it evenly with 1.5 times the weight of water, spray it into the granulated granules to coat the granules;
[0083] (6) Dry the coa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hygroscopicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com